A widely distributed gene cluster compensates for uricase loss in hominids
- PMID: 37541197
- PMCID: PMC10421625
- DOI: 10.1016/j.cell.2023.06.010
A widely distributed gene cluster compensates for uricase loss in hominids
Erratum in
-
A widely distributed gene cluster compensates for uricase loss in hominids.Cell. 2023 Sep 28;186(20):4472-4473. doi: 10.1016/j.cell.2023.08.036. Cell. 2023. PMID: 37774682 Free PMC article.
Abstract
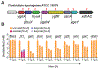
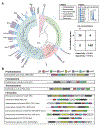
Approximately 15% of US adults have circulating levels of uric acid above its solubility limit, which is causally linked to the disease gout. In most mammals, uric acid elimination is facilitated by the enzyme uricase. However, human uricase is a pseudogene, having been inactivated early in hominid evolution. Though it has long been known that uric acid is eliminated in the gut, the role of the gut microbiota in hyperuricemia has not been studied. Here, we identify a widely distributed bacterial gene cluster that encodes a pathway for uric acid degradation. Stable isotope tracing demonstrates that gut bacteria metabolize uric acid to xanthine or short chain fatty acids. Ablation of the microbiota in uricase-deficient mice causes severe hyperuricemia, and anaerobe-targeted antibiotics increase the risk of gout in humans. These data reveal a role for the gut microbiota in uric acid excretion and highlight the potential for microbiome-targeted therapeutics in hyperuricemia.
Keywords: gout; hyperuricemia; microbiome; microbiota; uric acid; uricase.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.D. is a co-founder of Federation Bio. M.H.M. is a member of the Scientific Advisory Board of Hexagon Bio and co-founder of Design Pharmaceuticals.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

