Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer
- PMID: 37541199
- PMCID: PMC10414761
- DOI: 10.1016/j.cell.2023.07.004
Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer
Erratum in
-
Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer.Cell. 2024 Feb 15;187(4):1016. doi: 10.1016/j.cell.2024.01.016. Cell. 2024. PMID: 38364782 Free PMC article. No abstract available.
Abstract
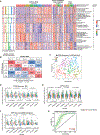
To improve the understanding of chemo-refractory high-grade serous ovarian cancers (HGSOCs), we characterized the proteogenomic landscape of 242 (refractory and sensitive) HGSOCs, representing one discovery and two validation cohorts across two biospecimen types (formalin-fixed paraffin-embedded and frozen). We identified a 64-protein signature that predicts with high specificity a subset of HGSOCs refractory to initial platinum-based therapy and is validated in two independent patient cohorts. We detected significant association between lack of Ch17 loss of heterozygosity (LOH) and chemo-refractoriness. Based on pathway protein expression, we identified 5 clusters of HGSOC, which validated across two independent patient cohorts and patient-derived xenograft (PDX) models. These clusters may represent different mechanisms of refractoriness and implicate putative therapeutic vulnerabilities.
Keywords: chemorefractory; high-grade serous ovarian cancer; machine learning; mass spectrometry; multiple reaction monitoring; platinum; precision oncology; predictive biomarker; prognostic biomarker; proteogenomic.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.J.B. has participated in advisory boards for the following companies: Clovis, Astra Zeneca, and GSK-Tesaro. A.N.H. is the Associate Editor of Clinical Chemistry and has a financial interest in the company Seattle Genetics. A.G.P. is the founder of Precision Assays, LLC. A.B.B. is employed by Synapse. S.Y. is an employee and shareholder of Sema4. B.J.W. has stock interests in Verastem and Exact Sciences.
Figures






References
-
- Bast RC Jr., Matulonis UA, Sood AK, Ahmed AA, Amobi AE, Balkwill FR, Wielgos-Bonvallet M, Bowtell DDL, Brenton JD, and Brugge JS (2019). Critical questions in ovarian cancer research and treatment: report of an American Association for Cancer Research Special Conference. Cancer 125, 1963–1972. - PMC - PubMed
-
- Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MKB, Ehlen T, Jayson GC, Johnson N, Swart AM, and Verheijen R (2018). Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 19, 1680–1687. - PubMed
-
- Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, and Karlan BY (2003). Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 97, 2187–2195. - PubMed
-
- Muggia FM (2003). Sequential single agents as first-line chemotherapy for ovarian cancer: a strategy derived from the results of GOG-132. Int. J. Gynecol. Cancer 13, 156–162. - PubMed
Publication types
MeSH terms
Grants and funding
- U24 CA210954/CA/NCI NIH HHS/United States
- U24 CA271037/CA/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- U24 CA210967/CA/NCI NIH HHS/United States
- S10 OD028685/OD/NIH HHS/United States
- R50 CA211499/CA/NCI NIH HHS/United States
- U24 CA210972/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- U24 CA210993/CA/NCI NIH HHS/United States
- P30 CA240139/CA/NCI NIH HHS/United States
- U01 CA214114/CA/NCI NIH HHS/United States
- U24 CA271114/CA/NCI NIH HHS/United States
- P50 CA136393/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

