Surgical versus non-surgical management for patients with malignant bowel obstruction (S1316): a pragmatic comparative effectiveness trial
- PMID: 37541263
- PMCID: PMC10530384
- DOI: 10.1016/S2468-1253(23)00191-7
Surgical versus non-surgical management for patients with malignant bowel obstruction (S1316): a pragmatic comparative effectiveness trial
Erratum in
-
Correction to Lancet Gastroenterol Hepatol 2023; 8: 908-18.Lancet Gastroenterol Hepatol. 2024 Feb;9(2):e2. doi: 10.1016/S2468-1253(23)00459-4. Lancet Gastroenterol Hepatol. 2024. PMID: 38215783 Free PMC article. No abstract available.
Abstract
Background: Malignant small bowel obstruction has a poor prognosis and is associated with multiple related symptoms. The optimal treatment approach is often unclear. We aimed to compare surgical versus non-surgical management with the aim to determine the optimal approach for managing malignant bowel obstruction.
Methods: S1316 was a pragmatic comparative effectiveness trial done within the National Cancer Trials Network at 30 hospital and cancer research centres in the USA, Mexico, Peru, and Colombia. Participants had an intra-abdominal or retroperitoneal primary cancer confirmed via pathological report and malignant bowel disease; were aged 18 years or older with a Zubrod performance status 0-2 within 1 week before admission; had a surgical indication; and treatment equipoise. Participants were randomly assigned (1:1) to surgical or non-surgical treatment using a dynamic balancing algorithm, balancing on primary tumour type. Patients who declined consent for random assignment were offered a prospective observational patient choice pathway. The primary outcome was the number of days alive and out of the hospital (good days) at 91 days. Analyses were based on intention-to-treat linear, logistic, and Cox regression models combining data from both pathways and adjusting for potential confounders. Treatment complications were assessed in all analysed patients in the study. This completed study is registered with ClinicalTrials.gov, NCT02270450.
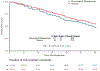
Findings: From May 11, 2015, to April 27, 2020, 221 patients were enrolled (143 [65%] were female and 78 [35%] were male). There were 199 evaluable participants: 49 in the randomised pathway (24 surgery and 25 non-surgery) and 150 in the patient choice pathway (58 surgery and 92 non-surgery). No difference was seen between surgery and non-surgery for the primary outcome of good days: mean 42·6 days (SD 32·2) in the randomised surgery group, 43·9 days (29·5) in the randomised non-surgery group, 54·8 days (27·0) in the patient choice surgery group, and 52·7 days (30·7) in the patient choice non-surgery group (adjusted mean difference 2·9 additional good days in surgical versus non-surgical treatment [95% CI -5·5 to 11·3]; p=0·50). During their initial hospital stay, six participants died, five due to cancer progression (four patients from the randomised pathway, two in each treatment group, and one from the patient choice pathway, in the surgery group) and one due to malignant bowel obstruction treatment complications (patient choice pathway, non-surgery). The most common grade 3-4 malignant bowel obstruction treatment complication was anaemia (three [6%] patients in the randomised pathway, all in the surgical group, and five [3%] patients in the patient choice pathway, four in the surgical group and one in the non-surgical group).
Interpretation: In our study, whether patients received a surgical or non-surgical treatment approach did not influence good days during the first 91 days after registration. These findings should inform treatment decisions for patients hospitalised with malignant bowel obstruction.
Funding: Agency for Healthcare Research and Quality and the National Cancer Institute.
Translation: For the Spanish translation of the abstract see Supplementary Materials section.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests GLA received payments to Fred Hutch for support for the present manuscript. AAS has grants or contracts from AbbVie, Aravive, AstraZeneca, Boehringer, Ingelheim, Clovis, Eisai, Ellipses, Immunogen, Merck, Oncoquest, Roche/Genentech, SeagenInc, TapImmune, Tesaro/GSK, VBL Therapeutics, and National Cancer Trial Network; has received honoraria for educational presentations or lectures from @Point of Care, Clinical Care Optios, Curio Science, Peerview, Bio ASCEND, RTP, and GOG Foundation; has received honorarium from Myriad; and has received support for attending meetings or travel from GOG, Society of Gynecologic Oncology, and NRG. DCF received support for the present manuscript in the form of payments through S1316 to Valley Health for patient enrolment; received payment or honoraria for lectures from Intuitive for the resident lecture programme; and has stock options in Intuitive. JDW has grants or contracts from Merck; receives royalties or licenses from UptoDate; receives payments for expert testimony from Medicolegal for consulting on gynaecological cancer; is a journal editor for the American College of Obstetricians and Gynecologists. BFa received visiting professor honoraria in April, 2022, at the Ohio State University Department of Surgery, October, 2021, at the University of Nebraska Department of Surgery, and December, 2020, at the University of Tennessee Department of Surgery; and has stock or stock options in Align Tech, Biogen, Bristol Myers Squibb, DexCom, Editas Medicine, Fulgent Genetics, GoodRx Holdings, Guardant Health, Globus Medical, Healthequity, HCA Healthcare, IDEXX Laboratories, Illumina, Intuitive Surgical, Invitae, Masimo, Moderna, Neurocrine Bioscience, Novocure, Quidel, Repligen, Seagen, Shockwave Medical, STAAR Surgical, UnitedHealth Group, Veeva Systems, Teladoc Health, and ResMed. All other authors declare no competing interests.
Figures
Comment in
-
Ensuring patients with malignant bowel obstruction are central in research and clinical decisions.Lancet Gastroenterol Hepatol. 2023 Oct;8(10):863-864. doi: 10.1016/S2468-1253(23)00203-0. Epub 2023 Aug 1. Lancet Gastroenterol Hepatol. 2023. PMID: 37541264 No abstract available.
-
Comparable results of surgical versus non-surgical management for patients with malignant bowel obstruction-a lesson from S1316 study.Hepatobiliary Surg Nutr. 2024 Apr 3;13(2):304-306. doi: 10.21037/hbsn-23-606. Epub 2024 Mar 22. Hepatobiliary Surg Nutr. 2024. PMID: 38617481 Free PMC article. No abstract available.
References
-
- Badgwell BD, Smith K, Liu P, Bruera E, Curley SA, Cormier JN. Indicators of surgery and survival in oncology inpatients requiring surgical evaluation for palliation. Supportive Care Cancer 2009; 17:727—734. - PubMed
-
- Badgwell BD, Contreras C, Askew RL, Krouse RS, Feig B, Cormier JN. Radiographic and clinical factors associated with improved outcomes in advanced cancer patients with bowel obstruction. J Palliat Med 2011; Sept:14(9): 990—996. - PubMed
-
- Pujara D, Chiang YJ, Cormier JN, Bruera E, Badgwell B. Selective Approach for Patients with Advanced Malignancy and Gastrointestinal Obstruction. J Am Coll Surg 2017; 225(1):53, Epub 2017. May 5. - PubMed
-
- Higginson IJ, Sen-Gupta GJA: Place of care in advanced cancer: A qualitative systematic literature review of patient preferences. J Palliat Med 2000; 3(3): 287—300. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous