EGFR-Induced and c-Src-Mediated CD47 Phosphorylation Inhibits TRIM21-Dependent Polyubiquitylation and Degradation of CD47 to Promote Tumor Immune Evasion
- PMID: 37541303
- PMCID: PMC10520678
- DOI: 10.1002/advs.202206380
EGFR-Induced and c-Src-Mediated CD47 Phosphorylation Inhibits TRIM21-Dependent Polyubiquitylation and Degradation of CD47 to Promote Tumor Immune Evasion
Abstract
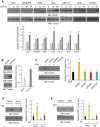
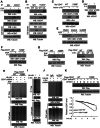
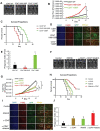
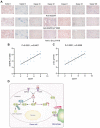
Tumor cells often overexpress immune checkpoint proteins, including CD47, for immune evasion. However, whether or how oncogenic activation of receptor tyrosine kinases, which are crucial drivers in tumor development, regulates CD47 expression is unknown. Here, it is demonstrated that epidermal growth factor receptor (EGFR) activation induces CD47 expression by increasing the binding of c-Src to CD47, leading to c-Src-mediated CD47 Y288 phosphorylation. This phosphorylation inhibits the interaction between the ubiquitin E3 ligase TRIM21 and CD47, thereby abrogating TRIM21-mediated CD47 K99/102 polyubiquitylation and CD47 degradation. Knock-in expression of CD47 Y288F reduces CD47 expression, increases macrophage phagocytosis of tumor cells, and inhibits brain tumor growth in mice. In contrast, knock-in expression of CD47 K99/102R elicits the opposite effects compared to CD47 Y288F expression. Importantly, CD47-SIRPα blockade with an anti-CD47 antibody treatment significantly enhances EGFR-targeted cancer therapy. In addition, CD47 expression levels in human glioblastoma (GBM) specimens correlate with EGFR and c-Src activation and aggravation of human GBM. These findings elucidate a novel mechanism underlying CD47 upregulation in EGFR-activated tumor cells and underscore the role of the EGFR-c-Src-TRIM21-CD47 signaling axis in tumor evasion and the potential to improve the current cancer therapy with a combination of CD47 blockade with EGFR-targeted remedy.
Keywords: CD47; TRIM21; c-Src; epidermal growth factor receptor; immune evasion; polyubiquitylation; tumor cells.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 82188102/National Natural Science Foundation of China
- 82030074/National Natural Science Foundation of China
- 81902151/National Natural Science Foundation of China
- 2020YFA0803300/Ministry of Science and Technology of the People's Republic of China
- LD21H160003/Zhejiang Natural Science Foundation-Key Project
- LY22H160012/Zhejiang Natural Science Foundation Grant
- 2019R01001/Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang
- 188020*194221901/029/Zhejiang University Research Fund
- Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A) in Hangzhou Medical College
- RS-2023-00208431/National Research Foundation of Korea (NRF) grant funded by the Korean government
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
