Administration of recombinant FOXN1 protein attenuates Alzheimer's pathology in mice
- PMID: 37541395
- PMCID: PMC10528256
- DOI: 10.1016/j.bbi.2023.07.027
Administration of recombinant FOXN1 protein attenuates Alzheimer's pathology in mice
Abstract
Background: Alzheimer's disease (AD) is the most common cause of dementia in older adults and characterized by progressive loss of memory and cognitive functions that are associated with amyloid-beta (Aβ) plaques and neurofibrillary tangles. Immune cells play an important role in the clearance of Aβ deposits and neurofibrillary tangles. T cells are the major component of the immune system. The thymus is the primary organ for T cell generation. T cell development in the thymus depends on thymic epithelial cells (TECs). However, TECs undergo both qualitative and quantitative loss over time. We have previously reported that a recombinant (r) protein containing FOXN1 and a protein transduction domain can increase the number of TECs and subsequently increases the number of T cells in mice. In this study we determined the ability of rFOXN1 to affect cognitive performance and AD pathology in mice.
Methods: Aged 3xTg-AD and APP/PS1 AD mice were injected with rFOXN1 or control protein. Cognitive performance, AD pathology, the thymic microenvironment and immune cells were then analyzed.
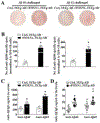
Results: Administration of rFOXN1 into AD mice improves cognitive performance and reduces Aβ plaque load and phosphorylated tau in the brain. This is related to rejuvenating the aged thymic microenvironment, which results in enhanced T cell generation in the thymus, leading to increased number of T cells, especially IFNγ-producing T cells, in the spleen and the choroid plexus (CP), enhanced expression of immune cell trafficking molecules in the CP, and increased migration of monocyte-derived macrophages into the brain. Furthermore, the production of anti-Aβ antibodies in the serum and the brain, and the macrophage phagocytosis of Aβ are enhanced in rFOXN1-treated AD mice.
Conclusions: Our results suggest that rFOXN1 protein has the potential to provide a novel approach to treat AD patients.
Keywords: Alzheimer’s disease; Amyloid-beta; FOXN1; T cells; Thymic epithelial cells; Thymus.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Administration of Amyloid Precursor Protein Gene Deleted Mouse ESC-Derived Thymic Epithelial Progenitors Attenuates Alzheimer's Pathology.Front Immunol. 2020 Aug 11;11:1781. doi: 10.3389/fimmu.2020.01781. eCollection 2020. Front Immunol. 2020. PMID: 32849642 Free PMC article.
-
Administration of anti-ERMAP antibody ameliorates Alzheimer's disease in mice.J Neuroinflammation. 2021 Nov 13;18(1):268. doi: 10.1186/s12974-021-02320-x. J Neuroinflammation. 2021. PMID: 34774090 Free PMC article.
-
Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice.Alzheimers Res Ther. 2021 Feb 9;13(1):40. doi: 10.1186/s13195-020-00761-9. Alzheimers Res Ther. 2021. PMID: 33563332 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
Cited by
-
Recombinant FOXN1 fusion protein increases T cell generation in old mice.Front Immunol. 2024 Jul 12;15:1423488. doi: 10.3389/fimmu.2024.1423488. eCollection 2024. Front Immunol. 2024. PMID: 39072332 Free PMC article.
-
Top 100 most-cited articles on tau protein: a bibliometric analysis and evidence mapping.Front Neurosci. 2024 Jan 31;18:1345225. doi: 10.3389/fnins.2024.1345225. eCollection 2024. Front Neurosci. 2024. PMID: 38356652 Free PMC article.
References
-
- 2022. 2022 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association 18, 700–789. - PubMed
-
- Anderson G, Takahama Y, 2012. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 33, 256–263. - PubMed
-
- Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E, Amit I, Schwartz M, 2016. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat. Med 22, 135–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

