Ceramides Increase Fatty Acid Utilization in Intestinal Progenitors to Enhance Stemness and Increase Tumor Risk
- PMID: 37541526
- PMCID: PMC10592225
- DOI: 10.1053/j.gastro.2023.07.017
Ceramides Increase Fatty Acid Utilization in Intestinal Progenitors to Enhance Stemness and Increase Tumor Risk
Abstract
Background & aims: Cancers of the alimentary tract, including esophageal adenocarcinomas, colorectal cancers, and cancers of the gastric cardia, are common comorbidities of obesity. Prolonged, excessive delivery of macronutrients to the cells lining the gut can increase one's risk for these cancers by inducing imbalances in the rate of intestinal stem cell proliferation vs differentiation, which can produce polyps and other aberrant growths. We investigated whether ceramides, which are sphingolipids that serve as a signal of nutritional excess, alter stem cell behaviors to influence cancer risk.
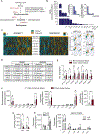
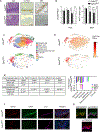
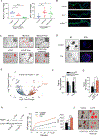
Methods: We profiled sphingolipids and sphingolipid-synthesizing enzymes in human adenomas and tumors. Thereafter, we manipulated expression of sphingolipid-producing enzymes, including serine palmitoyltransferase (SPT), in intestinal progenitors of mice, cultured organoids, and Drosophila to discern whether sphingolipids altered stem cell proliferation and metabolism.
Results: SPT, which diverts dietary fatty acids and amino acids into the biosynthetic pathway that produces ceramides and other sphingolipids, is a critical modulator of intestinal stem cell homeostasis. SPT and other enzymes in the sphingolipid biosynthesis pathway are up-regulated in human intestinal adenomas. They produce ceramides, which serve as prostemness signals that stimulate peroxisome-proliferator activated receptor-α and induce fatty acid binding protein-1. These actions lead to increased lipid utilization and enhanced proliferation of intestinal progenitors.
Conclusions: Ceramides serve as critical links between dietary macronutrients, epithelial regeneration, and cancer risk.
Keywords: Ceramides; Colorectal Cancer; Metabolism; Sphingolipids; Stem Cell.
Copyright © 2023 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interests.
SAS is a founder and shareholder of Centaurus Therapeutics. LPW is a shareholder of Centaurus Therapeutics. None of the other authors have relevant conflicts relevant to this manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD016232/OD/NIH HHS/United States
- R01 DK108833/DK/NIDDK NIH HHS/United States
- R01 DK115824/DK/NIDDK NIH HHS/United States
- T32 HL139451/HL/NHLBI NIH HHS/United States
- R44 DK116450/DK/NIDDK NIH HHS/United States
- R43 DK116450/DK/NIDDK NIH HHS/United States
- R35 GM140900/GM/NIGMS NIH HHS/United States
- U01 CA272529/CA/NCI NIH HHS/United States
- R01 DK131609/DK/NIDDK NIH HHS/United States
- R01 DK130296/DK/NIDDK NIH HHS/United States
- R01 DK112826/DK/NIDDK NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
- S10 OD018210/OD/NIH HHS/United States
- S10 RR024761/RR/NCRR NIH HHS/United States
- R01 DK122001/DK/NIDDK NIH HHS/United States
- R01 DK124326/DK/NIDDK NIH HHS/United States
- T32 DK091317/DK/NIDDK NIH HHS/United States
- S10 OD021505/OD/NIH HHS/United States
- R01 CA228346/CA/NCI NIH HHS/United States
- F99 CA253744/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

