A multilevel health system intervention for virological suppression in adolescents and young adults living with HIV in rural Kenya and Uganda (SEARCH-Youth): a cluster randomised trial
- PMID: 37541706
- PMCID: PMC11158418
- DOI: 10.1016/S2352-3018(23)00118-2
A multilevel health system intervention for virological suppression in adolescents and young adults living with HIV in rural Kenya and Uganda (SEARCH-Youth): a cluster randomised trial
Abstract
Background: Social and cognitive developmental events can disrupt care and medication adherence among adolescents and young adults living with HIV in sub-Saharan Africa. We hypothesised that a dynamic multilevel health system intervention helping adolescents and young adults and their providers navigate life-stage related events would increase virological suppression compared with standard care.
Methods: We did a cluster randomised, open-label trial of young individuals aged 15-24 years with HIV and receiving care in eligible clinics (operated by the government and with ≥25 young people receiving care) in rural Kenya and Uganda. After clinic randomisation stratified by region, patient population, and previous participation in the SEARCH trial, participants in intervention clinics received life-stage-based assessment at routine visits, flexible clinic access, and rapid viral load feedback. Providers had a secure mobile platform for interprovider consultation. The control clinics followed standard practice. The primary, prespecified endpoint was virological suppression (HIV RNA <400 copies per mL) at 2 years of follow-up among participants who enrolled before Dec 1, 2019, and received care at the study clinics. This trial is registered with ClinicalTrials.gov, NCT03848728, and is closed to recruitment.
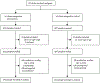
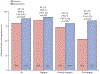
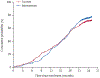
Findings: 28 clinics were enrolled and randomly assigned (14 control, 14 intervention) in January, 2019. Between March 14, 2019, and Nov 26, 2020, we recruited 1988 participants at the clinics, of whom 1549 were included in the analysis (785 at intervention clinics and 764 at control clinics). The median participant age was 21 years (IQR 19-23) and 1248 (80·6%) of 1549 participants were female. The mean proportion of participants with virological suppression at 2 years was 88% (95% CI 85-92) for participants in intervention clinics and 80% (77-84) for participants in control clinics, equivalent to a 10% beneficial effect of the intervention (risk ratio [RR] 1·10, 95% CI 1·03-1·16; p=0·0019). The intervention resulted in increased virological suppression within all subgroups of sex, age, and care status at baseline, with greatest improvement among those re-engaging in care (RR 1·60, 95% CI 1·00-2·55; p=0·025).
Interpretation: Routine and systematic life-stage-based assessment, prompt adherence support with rapid viral load testing, and patient-centred, flexible clinic access could help bring adolescents and young adults living with HIV closer towards a goal of universal virological suppression.
Funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development, US National Institutes of Health.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DH has received research grants from the National Institutes of Health, and non-financial support from Gilead and AbbVie, all via her institution. EC has received payments for consultation on unrelated studies of HIV and hypertension from the Infectious Diseases Research Collaboration in Uganda. All other authors declare no competing interests.
Figures
Comment in
-
Multilevel interventions for young people with HIV.Lancet HIV. 2023 Aug;10(8):e489-e490. doi: 10.1016/S2352-3018(23)00138-8. Lancet HIV. 2023. PMID: 37541704 No abstract available.
Similar articles
-
Point-of-care HIV viral load and targeted drug resistance mutation testing versus standard care for Kenyan children on antiretroviral therapy (Opt4Kids): an open-label, randomised controlled trial.Lancet Child Adolesc Health. 2022 Oct;6(10):681-691. doi: 10.1016/S2352-4642(22)00191-2. Epub 2022 Aug 18. Lancet Child Adolesc Health. 2022. PMID: 35987208 Free PMC article. Clinical Trial.
-
Financial incentives for achieving and maintaining viral suppression among HIV-positive adults in Uganda: a randomised controlled trial.Lancet HIV. 2019 Mar;6(3):e155-e163. doi: 10.1016/S2352-3018(18)30330-8. Epub 2019 Jan 16. Lancet HIV. 2019. PMID: 30660594 Free PMC article. Clinical Trial.
-
Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial.Lancet Glob Health. 2020 Feb;8(2):e264-e275. doi: 10.1016/S2214-109X(19)30526-1. Epub 2020 Jan 7. Lancet Glob Health. 2020. PMID: 31924539
-
Effect of universal HIV testing and treatment on socioeconomic wellbeing in rural Kenya and Uganda: a cluster-randomised controlled trial.Lancet Glob Health. 2022 Jan;10(1):e96-e104. doi: 10.1016/S2214-109X(21)00458-7. Lancet Glob Health. 2022. PMID: 34919862 Clinical Trial.
-
Adolescents and young adults with HIV and unsuppressed viral load: where do we go from here?Curr Opin HIV AIDS. 2024 Nov 1;19(6):368-376. doi: 10.1097/COH.0000000000000880. Epub 2024 Jul 29. Curr Opin HIV AIDS. 2024. PMID: 39145824 Review.
Cited by
-
CROI 2024: The Challenges of Sustained Viral Suppression, Advanced HIV Disease, and Ending the HIV Epidemic Targets.Top Antivir Med. 2024 Aug 12;32(4):542-567. Top Antivir Med. 2024. PMID: 39746674 Free PMC article. Review.
-
Clinical Implications of HIV Treatment and Prevention for Polygamous Families in Kenya and Uganda: "My Co-Wife Is the One Who Used to Encourage Me".J Int Assoc Provid AIDS Care. 2024 Jan-Dec;23:23259582241255171. doi: 10.1177/23259582241255171. J Int Assoc Provid AIDS Care. 2024. PMID: 38751360 Free PMC article.
-
Treatment of Advanced HIV in the Modern Era.Drugs. 2025 Jul;85(7):883-909. doi: 10.1007/s40265-025-02181-1. Epub 2025 May 12. Drugs. 2025. PMID: 40354016 Free PMC article. Review.
-
Paediatric antiretroviral therapy challenges with emerging integrase resistance.Curr Opin HIV AIDS. 2024 Nov 1;19(6):323-329. doi: 10.1097/COH.0000000000000876. Epub 2024 Jul 5. Curr Opin HIV AIDS. 2024. PMID: 38967797 Free PMC article. Review.
-
Determinants of implementation of a stepped care intervention for adolescents and youth living with HIV in Kenya: a qualitative evaluation.BMC Health Serv Res. 2025 May 15;25(1):702. doi: 10.1186/s12913-025-12875-7. BMC Health Serv Res. 2025. PMID: 40369586 Free PMC article. Clinical Trial.
References
-
- UNICEF. Global and regional trends. 2021. https://data.unicef.org/topic/adolescents/hiv-aids/ (accessed June 13, 2022).
-
- Inzaule SC, Kroeze S, Kityo CM, et al. Long-term HIV treatment outcomes and associated factors in sub-Saharan Africa: multicountry longitudinal cohort analysis. AIDS 2022; 36: 1437–47. - PubMed
-
- Sawyer SM, Afifi RA, Bearinger LH, et al. Adolescence: a foundation for future health. Lancet 2012; 379: 1630–40. - PubMed
-
- UNAIDS. Get on the fast-track: the life-cycle approach to HIV. Finding solutions for everyone at every stage of life. 2016. http://www.unaids.org/sites/default/files/media_asset/Get-on-the-Fast-Tr... (accessed June 13, 2022).
-
- Green LW, Kreuter MW, Deeds SG, Partrige KB. Health education planning: a diagnostic approach. Palo Alto, CA: Mayfield, 1980.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials