Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants
- PMID: 37542025
- PMCID: PMC10403593
- DOI: 10.1038/s41541-023-00708-9
Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants
Abstract
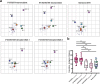
We report SARS-CoV-2 neutralizing antibody titers in sera of triple-vaccinated individuals who received a booster dose of an original monovalent or a bivalent BA.1- or BA.4/BA.5-adapted vaccine or had a breakthrough infection with Omicron variants BA.1, BA.2 or BA.4/BA.5. A bivalent BA.4/BA.5 booster or Omicron-breakthrough infection induced increased Omicron-neutralization titers compared with the monovalent booster. The XBB.1.5 variant effectively evaded neutralizing-antibody responses elicited by current vaccines and/or infection with previous variants.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Addetia, A. et al. Therapeutic and vaccine-induced cross-reactive antibodies with effector function against emerging Omicron variants. Preprint at bioRxiv10.1101/2023.01.17.523798 (2023).
LinkOut - more resources
Full Text Sources
Miscellaneous

