Exosomal PGAM1 promotes prostate cancer angiogenesis and metastasis by interacting with ACTG1
- PMID: 37542027
- PMCID: PMC10403531
- DOI: 10.1038/s41419-023-06007-4
Exosomal PGAM1 promotes prostate cancer angiogenesis and metastasis by interacting with ACTG1
Abstract
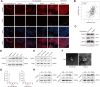
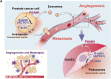
Tumor-derived exosomes and their contents promote cancer metastasis. Phosphoglycerate mutase 1 (PGAM1) is involved in various cancer-related processes. Nevertheless, the underlying mechanism of exosomal PGAM1 in prostate cancer (PCa) metastasis remains unclear. In this study, we performed in vitro and in vivo to determine the functions of exosomal PGAM1 in the angiogenesis of patients with metastatic PCa. We performed Glutathione-S-transferase pulldown, co-immunoprecipitation, western blotting and gelatin degradation assays to determine the pathway mediating the effect of exosomal PGAM1 in PCa. Our results revealed a significant increase in exosomal PGAM1 levels in the plasma of patients with metastatic PCa compared to patients with non-metastatic PCa. Furthermore, PGAM1 was a key factor initiating PCa cell metastasis by promoting invadopodia formation and could be conveyed by exosomes from PCa cells to human umbilical vein endothelial cells (HUVECs). In addition, exosomal PGAM1 could bind to γ-actin (ACTG1), which promotes podosome formation and neovascular sprouting in HUVECs. In vivo results revealed exosomal PGAM1 enhanced lung metastasis in nude mice injected with PCa cells via the tail vein. In summary, exosomal PGAM1 promotes angiogenesis and could be used as a liquid biopsy marker for PCa metastasis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

