Species-specific metabolites mediate host selection and larval recruitment of the symbiotic seastar shrimp
- PMID: 37542089
- PMCID: PMC10403617
- DOI: 10.1038/s41598-023-39527-2
Species-specific metabolites mediate host selection and larval recruitment of the symbiotic seastar shrimp
Abstract
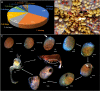
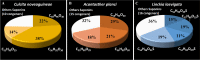
In marine environments, host selection, defining how symbiotic organisms recognize and interact with their hosts, is often mediated by olfactory communication. Although adult symbionts may select their hosts detecting chemosensory cues, no information is available concerning the recruitment of symbiotic larvae which is a crucial step to sustain symbioses over generations. This study investigates the olfactory recognition of seastar hosts by adult Zenopontonia soror shrimps and the recruitment of their larvae. We examine the semiochemicals that influence host selection using chemical extractions, behavioural experiments in olfactometers, and mass spectrometry analyses. After describing the symbiotic population and the embryonic development of shrimps, our results demonstrate that asterosaponins, which are traditionally considered as chemical defences in seastars, are species-specific and play a role in attracting the symbiotic shrimps. Adult shrimps were found to be attracted only by their original host species Culcita novaeguineae, while larvae were attracted by different species of seastars. This study provides the first chemical identification of an olfactory cue used by larvae of symbiotic organisms to locate their host for recruitment. These findings highlight the importance of chemical communication in the mediation of symbiotic associations, which has broader significant implications for understanding the ecological dynamics of marine ecosystems.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Paracer S, Ahmadjian V. Symbiosis: An Introduction to Biological Associations. Oxford University Press; 2000.
-
- Parmentier E, Michel L. Boundary lines in symbiosis forms. Symbiosis. 2013;60:1–5.
-
- Trilles J-P, Hipeau-Jacquotte R. Treatise on Zoology—Anatomy, Taxonomy, Biology. The Crustacea. Brill Academic; 2012.
-
- Johnstone RA, Bshary R. From parasitism to mutualism: Partner control in asymmetric interactions. Ecol. Lett. 2002;5:634–639.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

