Long-term nusinersen treatment across a wide spectrum of spinal muscular atrophy severity: a real-world experience
- PMID: 37542300
- PMCID: PMC10401775
- DOI: 10.1186/s13023-023-02769-4
Long-term nusinersen treatment across a wide spectrum of spinal muscular atrophy severity: a real-world experience
Abstract
Background: Spinal muscular atrophy (SMA) is an autosomal recessive disorder caused by a biallelic mutation in the SMN1 gene, resulting in progressive muscle weakness and atrophy. Nusinersen is the first disease-modifying drug for all SMA types. We report on effectiveness and safety data from 120 adults and older children with SMA types 1c-3 treated with nusinersen.
Methods: Patients were evaluated with the Hammersmith Functional Motor Scale Expanded (HFMSE; n = 73) or the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND; n = 47). Additionally, the Revised Upper Limb Module (RULM) and 6-minute walk test (6MWT) were used in a subset of patients. Patients were followed for up to 30 months of nusinersen treatment (mean, SD; 23, 14 months). Subjective treatment outcomes were evaluated with the Patients Global Impression-Improvement (PGI-I) scale used in all patients or caregivers at each follow-up visit.
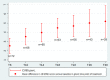
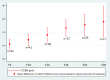
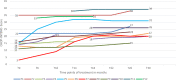
Results: An increase in the mean HFMSE score was noted at month 14 (T14) (3.9 points, p < 0.001) and month 30 (T30) (5.1 points, p < 0.001). The mean RULM score increased by 0.79 points at T14 (p = 0.001) and 1.96 points (p < 0.001) at month 30 (T30). The mean CHOP-INTEND increased by 3.6 points at T14 (p < 0.001) and 5.6 points at month 26 (p < 0.001). The mean 6MWT improved by 16.6 m at T14 and 27 m at T30 vs. baseline. A clinically meaningful improvement in HFMSE (≥ 3 points) was seen in 62% of patients at T14, and in 71% at T30; in CHOP INTEND (≥ 4 points), in 58% of patients at T14 and in 80% at T30; in RULM (≥ 2 points), in 26.6% of patients at T14 and in 43.5% at T30; and in 6MWT (≥ 30-meter increase), in 26% of patients at T14 and in 50% at T30. Improved PGI-I scores were reported for 75% of patients at T14 and 85% at T30; none of the patients reporting worsening at T30. Adverse events were mild and related to lumbar puncture.
Conclusions: In our study, nusinersen led to continuous functional improvement over 30-month follow-up and was well tolerated by adults and older children with a wide spectrum of SMA severity.
Keywords: Computed tomography–guided lumbar puncture; Functional tests; Nusinersen; Patient global impression – improvement; SMN2 gene; Scoliosis; Spinal muscular atrophy.
© 2023. Institut National de la Santé et de la Recherche Médicale (INSERM).
Conflict of interest statement
AL and AK-P received honoraria for advisory boards and speaking at educational events for Biogen, Novartis/AveXis, PTC and Roche; AL is a subinvestigator for SUNFISH and JEWELFISH studies; AK-P is a Principal Investigator for SUNFISH and JEWELFISH studies; AK-P and AL are involved in project supported with Institutional grant to Medical University of Warsaw from Biogen POL-SMG-17-11166 (5BIOGEN01); AW, AF, KA-G, ZG-W and RN received honoraria for speaking at educational events for Biogen and Roche; AP-Ch received honoraria for speaking at educational events for Biogen and PTC and is a subinvestigator in the DMD study; MB, PB, GR, DK, KM declare no conflict of interest.
Figures
References
-
- Tizzano E, Baiget M. Molecular basis of spinal muscular atrophy: the SMN gene. Neurologia. 2000;15:393–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

