Serum Proteomic Analysis of Peripartum Cardiomyopathy Reveals Distinctive Dysregulation of Inflammatory and Cholesterol Metabolism Pathways
- PMID: 37542511
- PMCID: PMC11974612
- DOI: 10.1016/j.jchf.2023.05.031
Serum Proteomic Analysis of Peripartum Cardiomyopathy Reveals Distinctive Dysregulation of Inflammatory and Cholesterol Metabolism Pathways
Abstract
Background: The pathophysiology of peripartum cardiomyopathy (PPCM) and its distinctive biological features remain incompletely understood. High-throughput serum proteomic profiling, a powerful tool to gain insights into the pathophysiology of diseases at a systems biology level, has never been used to investigate PPCM relative to nonischemic cardiomyopathy.
Objectives: The aim of this study was to characterize the pathophysiology of PPCM through serum proteomic analysis.
Methods: Aptamer-based proteomic analysis (SomaScan 7K) was performed on serum samples from women with PPCM (n = 67), women with nonischemic nonperipartum cardiomyopathy (NPCM) (n = 31), and age-matched healthy peripartum and nonperipartum women (n = 10 each). Serum samples were obtained from the IPAC (Investigation of Pregnancy-Associated Cardiomyopathy) and IMAC2 (Intervention in Myocarditis and Acute Cardiomyopathy) studies.
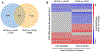
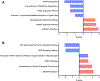
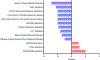
Results: Principal component analysis revealed unique clustering of each patient group (P for difference <0.001). Biological pathway analyses of differentially measured proteins in PPCM relative to NPCM, before and after normalization to pertinent healthy controls, highlighted specific dysregulation of inflammatory pathways in PPCM, including the upregulation of the cholesterol metabolism-related anti-inflammatory pathway liver-X receptor/retinoid-X receptor (LXR/RXR) (P < 0.01, Z-score 1.9-2.1). Cardiac recovery by 12 months in PPCM was associated with the downregulation of pro-inflammatory pathways and the upregulation of LXR/RXR, and an additional RXR-dependent pathway involved in the regulation of inflammation and metabolism, peroxisome proliferator-activated receptor α/RXRα signaling.
Conclusions: Serum proteomic profiling of PPCM relative to NPCM and healthy controls indicated that PPCM is a distinct disease entity characterized by the unique dysregulation of inflammation-related pathways and cholesterol metabolism-related anti-inflammatory pathways. These findings provide insight into the pathophysiology of PPCM and point to novel potential therapeutic targets.
Keywords: heart failure; inflammation; pregnancy; proteomics; serum proteomics.
Copyright © 2023 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This study has been funded through National Heart, Lung, and Blood Institute grants 5K08HLO145108-03 and 1R01HL160716-01 to Dr Adamo and T32-HL007227 to Dr Lovell, institutional funds from the Johns Hopkins Division of Cardiology awarded to Dr Adamo, and the Blumenthal Scholarship in Preventative Cardiology and American Heart Association 979462 funds awarded to Dr Sharma. Investigation of Pregnancy-Associated Cardiomyopathy was funded by National Institutes of Health grant HL102429 and IMAC2 by HL075038. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


Comment in
-
To Infinity and Beyond: Evolving Understanding of Peripartum Cardiomyopathy.JACC Heart Fail. 2023 Sep;11(9):1243-1245. doi: 10.1016/j.jchf.2023.06.035. Epub 2023 Aug 16. JACC Heart Fail. 2023. PMID: 37589614 No abstract available.
References
-
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010:767–778. 10.1093/eurjhf/hfq120 - DOI - PubMed
-
- Bauersachs J, König T, van der Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019;21(7):827–843. 10.1002/ejhf.1493 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

