Discovery of 1,3-disubstituted pyrazole peripheral cannabinoid receptor partial agonists
- PMID: 37543275
- PMCID: PMC10529378
- DOI: 10.1016/j.bmcl.2023.129430
Discovery of 1,3-disubstituted pyrazole peripheral cannabinoid receptor partial agonists
Abstract
Partial agonists of peripheral cannabinoid receptors (CBRs) have potential therapeutic applications in several medical conditions. However, (-)-trans-Δ9-tetrahydrocannabinol (THC), the principal active component of marijuana, which is a partial agonist of CB1 and CB2 penetrates the central nervous system (CNS) and produces adverse effects. Peripherally restricted partial agonists of CBRs, particularly of CB1, can be used to treat illnesses safely and effectively with a better therapeutic index. Here, we report on our efforts to synthesize pyrazole partial CBR agonists with peripheral selectivity, resulting in lead compound 40. This compound is a potent partial agonist of CB1 with ∼ 5-fold higher plasma biodistribution over brain and represents an early lead for optimization.
Keywords: Agonist; CB1; CB2; Cannabinoid; Compound; Peripheral.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
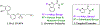
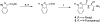
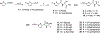
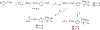

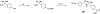
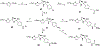
References
-
- Mouslech Z; Valla V, Endocannabinoid system: An overview of its potential in current medical practice. Neuro Endocrinol Lett 2009, 30 (2), 153–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

