Integrating metabolic expenditure information from wearable fitness sensors into an AI-augmented automated insulin delivery system: a randomised clinical trial
- PMID: 37543512
- PMCID: PMC10557965
- DOI: 10.1016/S2589-7500(23)00112-7
Integrating metabolic expenditure information from wearable fitness sensors into an AI-augmented automated insulin delivery system: a randomised clinical trial
Abstract
Background: Exercise can rapidly drop glucose in people with type 1 diabetes. Ubiquitous wearable fitness sensors are not integrated into automated insulin delivery (AID) systems. We hypothesised that an AID can automate insulin adjustments using real-time wearable fitness data to reduce hypoglycaemia during exercise and free-living conditions compared with an AID not automating use of fitness data.
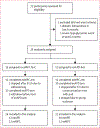
Methods: Our study population comprised of individuals (aged 21-50 years) with type 1 diabetes from from the Harold Schnitzer Diabetes Health Center clinic at Oregon Health and Science University, OR, USA, who were enrolled into a 76 h single-centre, two-arm randomised (4-block randomisation), non-blinded crossover study to use (1) an AID that detects exercise, prompts the user, and shuts off insulin during exercise using an exercise-aware adaptive proportional derivative (exAPD) algorithm or (2) an AID that automates insulin adjustments using fitness data in real-time through an exercise-aware model predictive control (exMPC) algorithm. Both algorithms ran on iPancreas comprising commercial glucose sensors, insulin pumps, and smartwatches. Participants executed 1 week run-in on usual therapy followed by exAPD or exMPC for one 12 h primary in-clinic session involving meals, exercise, and activities of daily living, and 2 free-living out-patient days. Primary outcome was time below range (<3·9 mmol/L) during the primary in-clinic session. Secondary outcome measures included mean glucose and time in range (3·9-10 mmol/L). This trial is registered with ClinicalTrials.gov, NCT04771403.
Findings: Between April 13, 2021, and Oct 3, 2022, 27 participants (18 females) were enrolled into the study. There was no significant difference between exMPC (n=24) versus exAPD (n=22) in time below range (mean [SD] 1·3% [2·9] vs 2·5% [7·0]) or time in range (63·2% [23·9] vs 59·4% [23·1]) during the primary in-clinic session. In the 2 h period after start of in-clinic exercise, exMPC had significantly lower mean glucose (7·3 [1·6] vs 8·0 [1·7] mmol/L, p=0·023) and comparable time below range (1·4% [4·2] vs 4·9% [14·4]). Across the 76 h study, both algorithms achieved clinical time in range targets (71·2% [16] and 75·5% [11]) and time below range (1·0% [1·2] and 1·3% [2·2]), significantly lower than run-in period (2·4% [2·4], p=0·0004 vs exMPC; p=0·012 vs exAPD). No adverse events occurred.
Interpretation: AIDs can integrate exercise data from smartwatches to inform insulin dosing and limit hypoglycaemia while improving glucose outcomes. Future AID systems that integrate exercise metrics from wearable fitness sensors may help people living with type 1 diabetes exercise safely by limiting hypoglycaemia.
Funding: JDRF Foundation and the Leona M and Harry B Helmsley Charitable Trust, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests PGJ and JRC have a financial interest in Pacific Diabetes Technologies, a company that might have a commercial interest in the results of this research and technology. JRC also reports advisory board participation for Zealand Pharma, Novo Nordisk, Insulet, and AstraZeneca. PGJ reports advisory board participation for Eli Lilly. PGJ and JRC have received research funding at their institution from Dexcom. All other authors declare no competing interests.
Figures

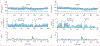
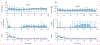
Comment in
-
Harnessing wearables and mobile phones to improve glycemic outcomes with automated insulin delivery.Lancet Digit Health. 2023 Sep;5(9):e548-e549. doi: 10.1016/S2589-7500(23)00127-9. Epub 2023 Aug 3. Lancet Digit Health. 2023. PMID: 37543513 No abstract available.
Similar articles
-
Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study.Lancet Diabetes Endocrinol. 2017 Apr;5(4):261-270. doi: 10.1016/S2213-8587(17)30001-3. Epub 2017 Jan 14. Lancet Diabetes Endocrinol. 2017. PMID: 28094136 Free PMC article. Clinical Trial.
-
Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial.Lancet Diabetes Endocrinol. 2016 Mar;4(3):233-243. doi: 10.1016/S2213-8587(15)00489-1. Epub 2016 Feb 3. Lancet Diabetes Endocrinol. 2016. PMID: 26850709 Free PMC article. Clinical Trial.
-
Flexible insulin therapy with a hybrid regimen of insulin degludec and continuous subcutaneous insulin infusion with pump suspension before exercise in physically active adults with type 1 diabetes (FIT Untethered): a single-centre, open-label, proof-of-concept, randomised crossover trial.Lancet Diabetes Endocrinol. 2020 Jun;8(6):511-523. doi: 10.1016/S2213-8587(20)30114-5. Lancet Diabetes Endocrinol. 2020. PMID: 32445738 Clinical Trial.
-
Glucose control and psychosocial outcomes with use of automated insulin delivery for 12 to 96 weeks in type 1 diabetes: a meta-analysis of randomised controlled trials.Diabetol Metab Syndr. 2023 Sep 28;15(1):190. doi: 10.1186/s13098-023-01144-4. Diabetol Metab Syndr. 2023. PMID: 37759290 Free PMC article. Review.
-
Overview of Artificial Intelligence-Driven Wearable Devices for Diabetes: Scoping Review.J Med Internet Res. 2022 Aug 9;24(8):e36010. doi: 10.2196/36010. J Med Internet Res. 2022. PMID: 35943772 Free PMC article.
Cited by
-
Leveraging AI-enhanced digital health with consumer devices for scalable cardiovascular screening, prediction, and monitoring.NPJ Cardiovasc Health. 2025;2(1):34. doi: 10.1038/s44325-025-00071-9. Epub 2025 Jul 2. NPJ Cardiovasc Health. 2025. PMID: 40620667 Free PMC article. Review.
-
NIH Fifth Artificial Pancreas Workshop 2023: Meeting Report: The Fifth Artificial Pancreas Workshop: Enabling Fully Automation, Access, and Adoption.J Diabetes Sci Technol. 2024 Jan;18(1):215-239. doi: 10.1177/19322968231201829. Epub 2023 Oct 9. J Diabetes Sci Technol. 2024. PMID: 37811866 Free PMC article.
-
The Future of Automated Insulin Delivery Systems.Endocr Pract. 2025 Jun 16:S1530-891X(25)00924-3. doi: 10.1016/j.eprac.2025.05.752. Online ahead of print. Endocr Pract. 2025. PMID: 40532759 Review.
-
The role of automated insulin delivery technology in diabetes.Diabetologia. 2024 Oct;67(10):2034-2044. doi: 10.1007/s00125-024-06165-w. Epub 2024 May 13. Diabetologia. 2024. PMID: 38740602 Free PMC article. Review.
-
Research Gaps, Challenges, and Opportunities in Automated Insulin Delivery Systems.J Diabetes Sci Technol. 2025 Jul;19(4):937-949. doi: 10.1177/19322968251338754. Epub 2025 Jul 1. J Diabetes Sci Technol. 2025. PMID: 40590464 Free PMC article. Review.
References
-
- Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016; 316: 1407–08. - PubMed
-
- Ware J, Boughton CK, Allen JM, et al. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health 2022; 4: e245–55. - PubMed
-
- Tsoukas MA, Majdpour D, Yale J-F, et al. A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: a single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit Health 2021; 3: e723–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

