Lysosomal acidification dysfunction in microglia: an emerging pathogenic mechanism of neuroinflammation and neurodegeneration
- PMID: 37543564
- PMCID: PMC10403868
- DOI: 10.1186/s12974-023-02866-y
Lysosomal acidification dysfunction in microglia: an emerging pathogenic mechanism of neuroinflammation and neurodegeneration
Abstract
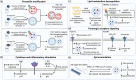
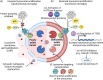
Microglia are the resident innate immune cells in the brain with a major role in orchestrating immune responses. They also provide a frontline of host defense in the central nervous system (CNS) through their active phagocytic capability. Being a professional phagocyte, microglia participate in phagocytic and autophagic clearance of cellular waste and debris as well as toxic protein aggregates, which relies on optimal lysosomal acidification and function. Defective microglial lysosomal acidification leads to impaired phagocytic and autophagic functions which result in the perpetuation of neuroinflammation and progression of neurodegeneration. Reacidification of impaired lysosomes in microglia has been shown to reverse neurodegenerative pathology in Alzheimer's disease. In this review, we summarize key factors and mechanisms contributing to lysosomal acidification impairment and the associated phagocytic and autophagic dysfunction in microglia, and how these defects contribute to neuroinflammation and neurodegeneration. We further discuss techniques to monitor lysosomal pH and therapeutic agents that can reacidify impaired lysosomes in microglia under disease conditions. Finally, we propose future directions to investigate the role of microglial lysosomal acidification in lysosome-mitochondria crosstalk and in neuron-glia interaction for more comprehensive understanding of its broader CNS physiological and pathological implications.
Keywords: Acidic nanoparticles; Autophagy; Cytokines; Lysosomal acidification; Neurodegenerative diseases; Neuroinflammation; Phagocytosis; Toxic protein aggregates.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Thion MS, Ginhoux F, Garel S. Microglia and early brain development: an intimate journey. Science. 2018;362:185–189. - PubMed
-
- Yang S, Qin C, Hu Z-W, Zhou L-Q, Yu H-H, Chen M, et al. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol Dis. 2021;152:105290. - PubMed
Publication types
MeSH terms
Grants and funding
- Presidential Postdoctoral Fellowship (Award Number: 021229-00001)/Nanyang Technological University
- Open Fund Young Investigator Research Grant (OF-YIRG) (Award Number: MOH-001147)/National Medical Research Council
- Dean's Postdoctoral Fellowship (Award Number: 021207-00001)/Lee Kong Chian School of Medicine, Nanyang Technological University
- Mistletoe Research Fellowship (Award Number: 022522-00001)/Momental Foundation
LinkOut - more resources
Full Text Sources
Medical

