Rituximab administration in pediatric patients with newly diagnosed acute lymphoblastic leukemia
- PMID: 37543655
- PMCID: PMC10666913
- DOI: 10.1038/s41375-023-01992-z
Rituximab administration in pediatric patients with newly diagnosed acute lymphoblastic leukemia
Abstract
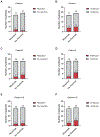
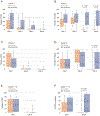
Polyethylene glycol (PEG)-asparaginase (pegaspargase) is a key agent in chemotherapy for acute lymphoblastic leukemia (ALL), but recipients frequently experience allergic reactions. We hypothesized that by decreasing antibody-producing CD20-positive B cells, rituximab may reduce these reactions. Children and adolescents (aged 1-18 years) with newly diagnosed B-ALL treated on the St. Jude Total XVII study were randomized to induction therapy with or without rituximab on day 3 (cohort 1) or on days 6 and 24 (cohort 2). Patient clinical demographics, CD20 expression, minimal residual disease (MRD), rituximab reactions, pegaspargase allergy, anti-pegaspargase antibodies, and pancreatitis were evaluated. Thirty-five patients received rituximab and 37 did not. Among the 35 recipients, 16 (45.7%) experienced a grade 2 or higher reaction to rituximab. There were no differences between recipients and non-recipients in the incidence of pegaspargase reactions (P > 0.999), anti-pegaspargase antibodies (P = 0.327), or pancreatitis (P = 0.480). CD20 expression on day 8 was significantly lower in rituximab recipients (P < 0.001), but there were no differences in MRD levels on day 8, 15, or at the end of induction. Rituximab administration during induction in pediatric patients with B-ALL was associated with a high incidence of infusion reactions with no significant decrease in pegaspargase allergies, anti-pegaspargase antibodies, or MRD.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
This work was funded partially by Servier Pharmaceuticals, LLC. The company had no role in the study design, the collection or analysis of data, or the decision to publish.
Figures

References
-
- Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373(16):1541–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

