IL-1β transgenic mouse model of inflammation driven esophageal and oral squamous cell carcinoma
- PMID: 37543673
- PMCID: PMC10404242
- DOI: 10.1038/s41598-023-39907-8
IL-1β transgenic mouse model of inflammation driven esophageal and oral squamous cell carcinoma
Abstract
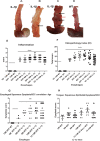
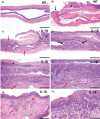
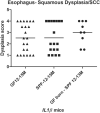
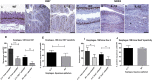
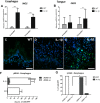
Chronic inflammation is integral to the development of esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC), although the latter has not been associated with reflux esophagitis. The L2-IL-1β transgenic mice, expressing human interleukin (IL)-1β in the oral, esophageal and forestomach squamous epithelia feature chronic inflammation and a stepwise development of Barrett's esophagus-like metaplasia, dysplasia and adenocarcinoma at the squamo-columnar junction. However, the functional consequences of IL-1β-mediated chronic inflammation in the oral and esophageal squamous epithelia remain elusive. We report for the first time that in addition to the previously described Barrett's esophagus-like metaplasia, the L2-IL-1β mice also develop squamous epithelial dysplasia with progression to squamous cell carcinoma (SCC) in the esophagus and the tongue. L2-IL-1β showed age-dependent progression of squamous dysplasia to SCC with approximately 40% (n = 49) and 23.5% (n = 17) incidence rates for esophageal and tongue invasive SCC respectively, by 12-15 months of age. Interestingly, SCC development and progression in L2-IL-1β was similar in both Germ Free (GF) and Specific Pathogen Free (SPF) conditions. Immunohistochemistry revealed a T cell predominant inflammatory profile with enhanced expression of Ki67, Sox2 and the DNA double-strand break marker, γ-H2AX, in the dysplastic squamous epithelia of L2-IL-1β mice. Pro-inflammatory cytokines, immunomodulatory players, chemoattractants for inflammatory cells (T cells, neutrophils, eosinophils, and macrophages) and oxidative damage marker, iNOS, were significantly increased in the esophageal and tongue tissues of L2-IL-1β mice. Our recent findings have expanded the translational utility of the IL-1β mouse model to aid in further characterization of the key pathways of inflammation driven BE and EAC as well as ESCC and Oral SCC.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wenig BM. Squamous cell carcinoma of the upper aerodigestive tract: Dysplasia and select variants. Mod. Pathol. 2017;30(s1):S112–S118. - PubMed
-
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88–95. - PubMed
-
- Reichenbach ZW, Murray MG, Saxena R, Farkas D, Karassik EG, Klochkova A, et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv. Cancer Res. 2019;144:95–135. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

