PP2A-B55alpha controls keratinocyte adhesion through dephosphorylation of the Desmoplakin C-terminus
- PMID: 37543698
- PMCID: PMC10404246
- DOI: 10.1038/s41598-023-37874-8
PP2A-B55alpha controls keratinocyte adhesion through dephosphorylation of the Desmoplakin C-terminus
Abstract
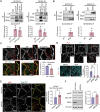
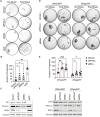
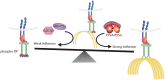
Critical for the maintenance of epidermal integrity and function are attachments between intermediate filaments (IF) and intercellular junctions called desmosomes. The desmosomal cytoplasmic plaque protein desmoplakin (DP) is essential for anchoring IF to the junction. DP-IF interactions are regulated by a phospho-regulatory motif within the DP C-terminus controlling keratinocyte intercellular adhesion. Here we identify the protein phosphatase 2A (PP2A)-B55α holoenzyme as the major serine/threonine phosphatase regulating DP's C-terminus and consequent intercellular adhesion. Using a combination of chemical and genetic approaches, we show that the PP2A-B55α holoenzyme interacts with DP at intercellular membranes in 2D- and 3D- epidermal models and human skin samples. Our experiments demonstrate that PP2A-B55α regulates the phosphorylation status of junctional DP and is required for maintaining strong desmosome-mediated intercellular adhesion. These data identify PP2A-B55α as part of a regulatory module capable of tuning intercellular adhesion strength and a candidate disease target in desmosome-related disorders of the skin and heart.
© 2023. Springer Nature Limited.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

