APE1 promotes radiation resistance against radiation-induced pyroptosis by inhibiting the STING pathway in lung adenocarcinoma
- PMID: 37544034
- PMCID: PMC10424251
- DOI: 10.1016/j.tranon.2023.101749
APE1 promotes radiation resistance against radiation-induced pyroptosis by inhibiting the STING pathway in lung adenocarcinoma
Abstract
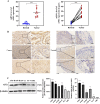
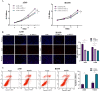
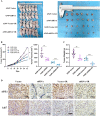
Mammalian apurinic/apyrimidinic endonuclease 1 (APE1, APEX1) is a multifunctional enzyme that maintains cellular homeostasis. It is involved in the base excision repair (BER) pathway and plays a key role in radiation-induced DNA damage response. However, the relationship between APE1-driven radiation resistance and pyroptosis in lung adenocarcinoma (LUAD) cells and the underlying molecular mechanisms remain unclear. We found that APE1 was significantly upregulated in LUAD tissues compared to para-carcinoma tissues and promoted the proliferation and invasion of LUAD cells in vitro and in vivo. Mechanistically, APE1 inhibited pyroptosis by inactivating the interferon gene stimulator (STING) pathway via direct interaction with AIM2 and DDX41, as detected by RNA-seq and co-immunoprecipitation. APE1 protects LUAD cells against radiation-induced damage and induces radio-resistance by targeting the STING pathway. It can induce pyroptosis and is negatively regulated by interactions with AIM2 and DDX41. Therefore, APE1 inhibitors should be considered to enhance the radiosensitivity of LUAD cells and improve patient prognosis and therapeutic outcomes. Thus, APE1 play a role in the tumor immune microenvironment and in tumor immunotherapy.
Keywords: APE1; LUAD; Pyroptosis; Radiosensitivity; STING.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest None.
Figures





Similar articles
-
RRM2 silencing suppresses malignant phenotype and enhances radiosensitivity via activating cGAS/STING signaling pathway in lung adenocarcinoma.Cell Biosci. 2021 Apr 15;11(1):74. doi: 10.1186/s13578-021-00586-5. Cell Biosci. 2021. PMID: 33858512 Free PMC article.
-
The role of the N-terminal domain of human apurinic/apyrimidinic endonuclease 1, APE1, in DNA glycosylase stimulation.DNA Repair (Amst). 2018 Apr;64:10-25. doi: 10.1016/j.dnarep.2018.02.001. Epub 2018 Feb 11. DNA Repair (Amst). 2018. PMID: 29475157
-
APE1 promotes lung adenocarcinoma through G4-mediated transcriptional reprogramming of urea cycle metabolism.iScience. 2025 Mar 25;28(5):112275. doi: 10.1016/j.isci.2025.112275. eCollection 2025 May 16. iScience. 2025. PMID: 40276763 Free PMC article.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
-
Research Progress in Human AP Endonuclease 1: Structure, Catalytic Mechanism, and Inhibitors.Curr Protein Pept Sci. 2022;23(2):77-88. doi: 10.2174/1389203723666220406132737. Curr Protein Pept Sci. 2022. PMID: 35388752 Review.
Cited by
-
Isoliensinine exerts antitumor effects in lung adenocarcinoma by inhibiting APEX1-driven ROS production.Front Pharmacol. 2025 May 27;16:1555802. doi: 10.3389/fphar.2025.1555802. eCollection 2025. Front Pharmacol. 2025. PMID: 40495893 Free PMC article.
-
Unveiling the therapeutic potential: KBU2046 halts triple-negative breast cancer cell migration by constricting TGF-β1 activation in vitro.Oncol Res. 2024 Oct 16;32(11):1791-1802. doi: 10.32604/or.2024.049348. eCollection 2024. Oncol Res. 2024. PMID: 39449805 Free PMC article.
-
DDX52 gene expression in LUAD tissues indicates potential as a prognostic biomarker and therapeutic target.Sci Rep. 2023 Oct 13;13(1):17434. doi: 10.1038/s41598-023-44347-5. Sci Rep. 2023. PMID: 37833424 Free PMC article.
-
Inhibition of non-small cell lung cancer metastasis by knocking down APE1 through regulating myeloid-derived suppressor cells-induced immune disorders.Aging (Albany NY). 2024 Jun 14;16(12):10435-10445. doi: 10.18632/aging.205938. Epub 2024 Jun 14. Aging (Albany NY). 2024. PMID: 38885059 Free PMC article.
-
Nuclear receptors as novel regulators that modulate cancer radiosensitivity and normal tissue radiotoxicity.Mol Cancer. 2025 May 30;24(1):155. doi: 10.1186/s12943-025-02362-2. Mol Cancer. 2025. PMID: 40442680 Free PMC article. Review.
References
-
- Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019;69(3):184–210. - PubMed
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71(1):7–33. - PubMed
-
- Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

