Safety, tolerability, and immunogenicity of the Ebola Sudan chimpanzee adenovirus vector vaccine (cAd3-EBO S) in healthy Ugandan adults: a phase 1, open-label, dose-escalation clinical trial
- PMID: 37544326
- PMCID: PMC10837320
- DOI: 10.1016/S1473-3099(23)00344-4
Safety, tolerability, and immunogenicity of the Ebola Sudan chimpanzee adenovirus vector vaccine (cAd3-EBO S) in healthy Ugandan adults: a phase 1, open-label, dose-escalation clinical trial
Erratum in
-
Correction to Lancet Infect Dis 2023; published online Aug 3. https://doi.org/10.1016/S1473-3099(23)00344-4.Lancet Infect Dis. 2023 Oct;23(10):e400. doi: 10.1016/S1473-3099(23)00554-6. Epub 2023 Aug 28. Lancet Infect Dis. 2023. PMID: 37652067 No abstract available.
Abstract
Background: Sudan Ebola virus can cause severe viral disease, with an average case fatality rate of 54%. A recent outbreak of Sudan Ebola virus in Uganda caused 55 deaths among 164 confirmed cases in the second half of 2022. Although vaccines and therapeutics specific for Zaire Ebola virus have been approved for use during outbreak situations, Sudan Ebola virus is an antigenically distinct virus with no approved vaccines available.
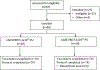
Methods: In this phase 1, open-label, dose-escalation trial we evaluated the safety, tolerability, and immunogenicity of a monovalent chimpanzee adenovirus 3 vaccine against Sudan Ebola virus (cAd3-EBO S) at Makerere University Walter Reed Project in Kampala, Uganda. Study participants were recruited from the Kampala metropolitan area using International Review Board-approved written and electronic media explaining the trial intervention. Healthy adults without previous receipt of Ebola, Marburg, or cAd3 vectored-vaccines were enrolled to receive cAd3-EBO S at either 1 × 1010 or 1 × 1011 particle units (PU) in a single intramuscular vaccination and were followed up for 48 weeks. Primary safety and tolerability endpoints were assessed in all vaccine recipients by reactogenicity for the first 7 days, adverse events for the first 28 days, and serious adverse events throughout the study. Secondary immunogenicity endpoints included evaluation of binding antibody and T-cell responses against the Sudan Ebola virus glycoprotein, and neutralising antibody responses against the cAd3 vector at 4 weeks after vaccination. This study is registered with ClinicalTrials.gov, NCT04041570, and is completed.
Findings: 40 healthy adults were enrolled between July 22 and Oct 1, 2019, with 20 receiving 1 × 1010 PU and 20 receiving 1 × 1011 PU of cAd3-EBO S. 38 (95%) participants completed all follow-up visits. The cAd3-EBO S vaccine was well tolerated with no severe adverse events. The most common reactogenicity symptoms were pain or tenderness at the injection site (34 [85%] of 40), fatigue (29 [73%] of 40), and headache (26 [65%] of 40), and were mild to moderate in severity. Positive responses for glycoprotein-specific binding antibodies were induced by 2 weeks in 31 (78%) participants, increased to 34 (85%) participants by 4 weeks, and persisted to 48 weeks in 31 (82%) participants. Most participants developed glycoprotein-specific T-cell responses (20 [59%, 95% CI 41-75] of 34; six participants were removed from the T cell analysis after failing quality control parameters) by 4 weeks after vaccination, and neutralising titres against the cAd3 vector were also increased from baseline (90% inhibitory concentration of 47, 95% CI 30-73) to 4 weeks after vaccination (196, 125-308).
Interpretation: The cAd3-EBO S vaccine was safe at both doses, rapidly inducing immune responses in most participants after a single injection. The rapid onset and durability of the vaccine-induced antibodies make this vaccine a strong candidate for emergency deployment in Sudan Ebola virus outbreaks.
Funding: National Institutes of Health via interagency agreement with Walter Reed Army Institute of Research.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests NJS is listed on patents involving cAd3-vectored vaccines. All other authors declare no competing interests.
Figures

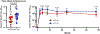
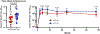
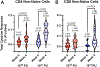
Comment in
-
Developing a vaccine against Sudan virus disease.Lancet Infect Dis. 2023 Dec;23(12):1333-1335. doi: 10.1016/S1473-3099(23)00360-2. Epub 2023 Aug 3. Lancet Infect Dis. 2023. PMID: 37544325 No abstract available.
References
-
- Centers for Disease Control and Prevention. Ebola Disease Distribution Map: Cases of Ebola Disease in Africa Since 1976. 2023 March 24 2023. https://www.cdc.gov/vhf/ebola/history/distribution-map.html (accessed 2023 April 21).
-
- Takada A. Ebolavirus and Other Filoviruses. In: Rezaei N, ed. Encylopedia of Infection and Immunity: Elsevier; 2022.
-
- World Health Organization. Disease Outbreak News; Ebola disease caused by Sudan ebolavirus – Uganda. Last Update 14 January 2023. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON433.
-
- Kuhn JH, Amarasinghe GK, & Perry DL Filoviridae. In: Knipe PMHDM, ed. Fields Virology: Emerging Viruses. 7th ed: Lippincott Williams & Wilkins; 2021: 449–503.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

