Hemophagocytic lymphohistiocytosis-like hyperinflammation due to a de novo mutation in DPP9
- PMID: 37544411
- PMCID: PMC7615848
- DOI: 10.1016/j.jaci.2023.07.013
Hemophagocytic lymphohistiocytosis-like hyperinflammation due to a de novo mutation in DPP9
Erratum in
-
Corrigendum.J Allergy Clin Immunol. 2025 Oct;156(4):1124. doi: 10.1016/j.jaci.2025.08.002. Epub 2025 Aug 27. J Allergy Clin Immunol. 2025. PMID: 40864024 No abstract available.
Abstract
Background: Genetic defects in components of inflammasomes can cause autoinflammation. Biallelic loss-of-function mutations in dipeptidyl peptidase 9 (DPP9), a negative regulator of the NLRP1 and CARD8 inflammasomes, have recently been shown to cause an inborn error of immunity characterized by pancytopenia, skin manifestations, and increased susceptibility to infections.
Objective: We sought to study the molecular basis of autoinflammation in a patient with severe infancy-onset hyperinflammation associated with signs of fulminant hemophagocytic lymphohistiocytosis.
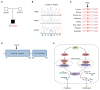
Methods: Using heterologous cell models as well as patient cells, we performed genetic, immunologic, and molecular investigations to identify the genetic cause and to assess the impact of the identified mutation on inflammasome activation.
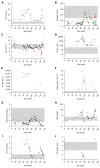
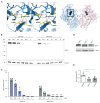
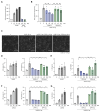
Results: The patient exhibited pancytopenia with decreased neutrophils and T, B, and natural killer cells, and markedly elevated levels of lactate dehydrogenase, ferritin, soluble IL-2 receptor, and triglycerides. In addition, serum levels of IL-1β and IL-18 were massively increased, consistent with inflammasome activation. Genetic analysis revealed a previously undescribed de novo mutation in DPP9 (c.755G>C, p.Arg252Pro) affecting a highly conserved amino acid residue. The mutation led to destabilization of the DPP9 protein as shown in transiently transfected HEK293T cells and in patient-derived induced pluripotent stem cells. Using functional inflammasome assays in HEK293T cells, we demonstrated that mutant DPP9 failed to restrain the NLRP1 and CARD8 inflammasomes, resulting in constitutive inflammasome activation. These findings suggest that the Arg252Pro DPP9 mutation acts in a dominant-negative manner.
Conclusions: A de novo mutation in DPP9 leads to severe infancy-onset autoinflammation because of unleashed inflammasome activation.
Keywords: CARD8; DPP9; IL-18; IL-1β; Inborn error of immunity; NLRP1; autoinflammation; hemophagocytic lymphohistiocytosis; inflammasome; proinflammatory cytokines.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no relevant conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

