Gold-Catalyzed Asymmetric Transformation of Hydroxylated Propargylic Esters
- PMID: 37544902
- PMCID: PMC11419700
- DOI: 10.1002/cplu.202300314
Gold-Catalyzed Asymmetric Transformation of Hydroxylated Propargylic Esters
Abstract
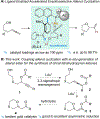
By combining tandem asymmetric gold catalysis and subsequent stereoconvergent hydrolysis of enol ester in a one-pot process, hydroxylated propargylic esters are converted into chiral β-oxygenated ketones with mostly good enantiomeric ratios and in largely good to excellent yields. The product chiral center is formed via stereoselective cyclization of a hydroxylated allenyl ester intermediate, which is enabled by asymmetric gold-ligand cooperation.
Keywords: catalysis; chiral; gold; ligand; metal-ligand cooperation.
© 2023 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interests
The authors declare no conflict of interest.
Figures
References
-
- Grützmacher H, Angew. Chem. Int. Ed 2008, 47, 1814–1818; - PubMed
- Askevold B, Roesky HW, Schneider S, ChemCatChem 2012, 4, 307–320;
- Khusnutdinova JR, Milstein D, Angew. Chem. Int. Ed 2015, 54, 12236–12273; - PubMed
- Trincado M, Grützmacher H, in Cooperative Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 2015, pp. 67–110.
-
- Cheng X, Zhang L, CCS Chem 2021, 3, 1989–2002.
-
- Wang Z, Nicolini C, Hervieu C, Wong Y-F, Zanoni G, Zhang L, J. Am. Chem. Soc 2017, 139, 16064–16067. - PubMed
-
- Sengupta S, Shi X, ChemCatChem 2010, 2, 609–619;
- Pradal A, Toullec PY, Michelet V, Synthesis 2011, 1501–1514;
- López F, Mascareñas JL, Beilstein J Org. Chem 2013, 9, 2250–2264; - PMC - PubMed
- Zi W, Dean Toste F, Chem. Soc. Rev 2016, 45, 4567–4589; - PubMed
- Li Y, Li W, Zhang J, Chem. Eur. J 2017, 23, 467–512; - PubMed
- Gutman K, Zhang L, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2023, pp. 10.1016/B1978-1010-1032-390644-390649.300095-390640. - DOI
-
- Wang S, Zhang G, Zhang L, Synlett 2010, 692–706;
- León Rojas AF, Kyne SH, Chan PWH, Acc. Chem. Res 2023, 56, 1406–1420. - PubMed