Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications
- PMID: 37544909
- PMCID: PMC10558674
- DOI: 10.1002/advs.202303326
Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications
Abstract
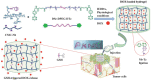
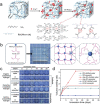
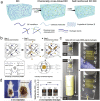
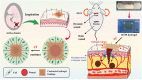
Hydrogels with tailor-made swelling-shrinkable properties have aroused considerable interest in numerous biomedical domains. For example, as swelling is a key issue for blood and wound extrudates absorption, the transference of nutrients and metabolites, as well as drug diffusion and release, hydrogels with high swelling capacity have been widely applicated in full-thickness skin wound healing and tissue regeneration, and drug delivery. Nevertheless, in the fields of tissue adhesives and internal soft-tissue wound healing, and bioelectronics, non-swelling hydrogels play very important functions owing to their stable macroscopic dimension and physical performance in physiological environment. Moreover, the negative swelling behavior (i.e., shrinkage) of hydrogels can be exploited to drive noninvasive wound closure, and achieve resolution enhancement of hydrogel scaffolds. In addition, it can help push out the entrapped drugs, thus promote drug release. However, there still has not been a general review of the constructions and biomedical applications of hydrogels from the viewpoint of swelling-shrinkable properties. Therefore, this review summarizes the tactics employed so far in tailoring the swelling-shrinkable properties of hydrogels and their biomedical applications. And a relatively comprehensive understanding of the current progress and future challenge of the hydrogels with different swelling-shrinkable features is provided for potential clinical translations.
Keywords: biomedical applications; high-swelling hydrogels; hydrogels; non-swelling hydrogels; shrinkable hydrogels.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















References
-
- Kaith B. S., Singh A., Sharma A. K., Sud D., J. Polym. Environ. 2021, 29, 3827.
-
- Vermonden T., Klumperman B., Eur. Polym. J. 2015, 72, 341.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
