Nanomedicine in cancer therapy
- PMID: 37544972
- PMCID: PMC10404590
- DOI: 10.1038/s41392-023-01536-y
Nanomedicine in cancer therapy
Abstract
Cancer remains a highly lethal disease in the world. Currently, either conventional cancer therapies or modern immunotherapies are non-tumor-targeted therapeutic approaches that cannot accurately distinguish malignant cells from healthy ones, giving rise to multiple undesired side effects. Recent advances in nanotechnology, accompanied by our growing understanding of cancer biology and nano-bio interactions, have led to the development of a series of nanocarriers, which aim to improve the therapeutic efficacy while reducing off-target toxicity of the encapsulated anticancer agents through tumor tissue-, cell-, or organelle-specific targeting. However, the vast majority of nanocarriers do not possess hierarchical targeting capability, and their therapeutic indices are often compromised by either poor tumor accumulation, inefficient cellular internalization, or inaccurate subcellular localization. This Review outlines current and prospective strategies in the design of tumor tissue-, cell-, and organelle-targeted cancer nanomedicines, and highlights the latest progress in hierarchical targeting technologies that can dynamically integrate these three different stages of static tumor targeting to maximize therapeutic outcomes. Finally, we briefly discuss the current challenges and future opportunities for the clinical translation of cancer nanomedicines.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures
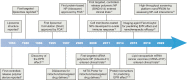
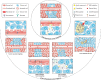
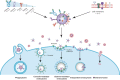
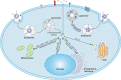
References
-
- Gotwals P, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. - PubMed
-
- Nam J, et al. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019;4:398–414.
-
- Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;8:660-IN610. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

