Clinico-histological and molecular features of hepatocellular carcinoma from nonalcoholic fatty liver disease
- PMID: 37545384
- PMCID: PMC10551597
- DOI: 10.1111/cas.15925
Clinico-histological and molecular features of hepatocellular carcinoma from nonalcoholic fatty liver disease
Abstract
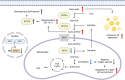
Patients with nonalcoholic fatty liver disease (NAFLD) continue to increase with the epidemics of obesity, and NAFLD is estimated to become the most prevalent etiology of hepatocellular carcinoma (HCC). Recently, NAFLD-HCC has been recognized to have clinico-histologically and molecularly distinct features from those from other etiologies, including a lower incidence rate of HCC and less therapeutic efficacy to immune checkpoint inhibitors (ICIs). Consistent with the clinical observations that up to 50% of NAFLD-HCC occurs in the absence of cirrhosis, the imbalance of pro- and antitumorigenic hepatic stellate cells termed as myHSC and cyHSC can contribute to the creation of an HCC-prone hepatic environment, independent of the absolute fibrosis abundance. Immune deregulations by accumulated metabolites in NAFLD-affected livers, such as a fatty-acid-induced loss of cytotoxic CD4 T cells serving for immune surveillance and "auto-aggressive" CXCR6+ CD8 T cells, may promote hepatocarcinogenesis and diminish therapeutic response to ICIs. Steatohepatitic HCC (SH-HCC), characterized by the presence of fat accumulation in tumor cells, ballooned tumor cells, Mallory-Denk body, interstitial fibrosis, and intratumor immune cell infiltration, may represent a metabolic reprogramming for adapting to a lipid-rich tumor microenvironment by downregulating CPT2 and leveraging its intermediates as an "oncometabolite." Genome-wide analyses suggested that SH-HCC may be more responsive to ICIs given its mutual exclusiveness with β-catenin mutation/activation that promotes immune evasion. Thus, further understanding of NAFLD-specific hepatocarcinogenesis and HCC would enable us to improve the current daily practice and eventually the prognoses of patients with NAFLD.
Keywords: nonalcoholic fatty liver disease; nonalcoholic steatohepatitis; noncirrhosis; steatohepatitic hepatocellular carcinoma; subtype.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
NH received honoraria from Taisho Pharmaceutical Holdings, Gilead Sciences, Abbvie, and Kowa Pharmaceuticals and grant support from Otsuka Pharmaceuticals. NF discloses no conflicts of interest.
Figures


Similar articles
-
Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH.Am J Surg Pathol. 2010 Nov;34(11):1630-6. doi: 10.1097/PAS.0b013e3181f31caa. Am J Surg Pathol. 2010. PMID: 20975341
-
Clinicopathologic features and prognosis of steatohepatitic hepatocellular carcinoma based on varying cutoffs of tumoral steatohepatitic changes.Am J Clin Pathol. 2025 Mar 8;163(3):411-418. doi: 10.1093/ajcp/aqae136. Am J Clin Pathol. 2025. PMID: 39418121
-
Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question.World J Gastroenterol. 2015 Apr 14;21(14):4103-10. doi: 10.3748/wjg.v21.i14.4103. World J Gastroenterol. 2015. PMID: 25892859 Free PMC article. Review.
-
Tumor Necrosis Factor-Alpha and Adiponectin in Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma.Cancers (Basel). 2023 Nov 6;15(21):5306. doi: 10.3390/cancers15215306. Cancers (Basel). 2023. PMID: 37958479 Free PMC article. Review.
-
Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.World J Gastroenterol. 2014 Nov 14;20(42):15539-48. doi: 10.3748/wjg.v20.i42.15539. World J Gastroenterol. 2014. PMID: 25400438 Free PMC article. Review.
Cited by
-
AI-based phenotyping of hepatic fiber morphology to inform molecular alterations in metabolic dysfunction-associated steatotic liver disease.Hepatology. 2025 Apr 22:10.1097/HEP.0000000000001360. doi: 10.1097/HEP.0000000000001360. Online ahead of print. Hepatology. 2025. PMID: 40262132
-
Lipogenesis and MASLD: re-thinking the role of SREBPs.Arch Toxicol. 2025 Jun;99(6):2299-2312. doi: 10.1007/s00204-025-04052-w. Epub 2025 May 6. Arch Toxicol. 2025. PMID: 40327083 Review.
-
Examination of the causal role of immune cells in non-alcoholic fatty liver disease by a bidirectional Mendelian randomization study.Open Med (Wars). 2025 Feb 19;20(1):20251154. doi: 10.1515/med-2025-1154. eCollection 2025. Open Med (Wars). 2025. PMID: 39989616 Free PMC article.
-
Squalene Epoxidase: Its Regulations and Links with Cancers.Int J Mol Sci. 2024 Mar 30;25(7):3874. doi: 10.3390/ijms25073874. Int J Mol Sci. 2024. PMID: 38612682 Free PMC article.
-
Combination Therapy of Immune Checkpoint Inhibitors with Locoregional Therapy for Hepatocellular Carcinoma.Cancers (Basel). 2023 Oct 20;15(20):5072. doi: 10.3390/cancers15205072. Cancers (Basel). 2023. PMID: 37894439 Free PMC article. Review.
References
-
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537‐2564. - PubMed
-
- Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672‐2682. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

