Anti-interleukin 4 receptor α antibody for the treatment of Chinese bullous pemphigoid patients with diverse comorbidities and a 1-year follow-up: a monocentric real-world study
- PMID: 37545503
- PMCID: PMC10399451
- DOI: 10.3389/fimmu.2023.1165106
Anti-interleukin 4 receptor α antibody for the treatment of Chinese bullous pemphigoid patients with diverse comorbidities and a 1-year follow-up: a monocentric real-world study
Abstract
Background: Bullous pemphigoid (BP) is a common subepidermal bullous disorder that lacks adequate treatment alternatives. Dupilumab, an anti-interleukin (IL) 4 receptor α antibody blocking Th2 molecules IL-4 and 13, has been used off-label and shown to be effective in refractory BP cases.
Methods: BP patients with various disease severities and comorbidities were included in this case series. All patients received dupilumab alone or in combination with immunosuppressants in a real-world setting. Complete remission (CR) was defined as the absence of pruritus symptoms and previous BP eruptions, with only hyperpigmentation patches and without newly occurring lesions for at least 4 weeks. Disease relapse was classified as the appearance of three or more new lesions within 1 month or at least one large urticarial or eczematous lesion that did not resolve within a week.
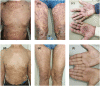
Findings: Ten individuals were enrolled in this case series. Pruritus symptoms and BP eruptions improved significantly in nine patients (90%). Seven patients (70%) attained CR, including all mild-to-moderate (100%) cases and three of six (50%) severe BP cases. At the dupilumab monotherapy stage, eosinophilia was observed in two severe cases. One patient out of seven (14.3%) relapsed after 1 year of follow-up after CR.
Conclusion: Treatment of BP with diverse comorbidities with anti-IL-4 receptor α antibody provides further credentials to a prospective randomized study. More impressive efficacy and safety profiles were observed in patients with mild-to-moderate disease after 1 year of follow-up. Eosinophilia may occur in patients receiving dupilumab monotherapy.
Keywords: Bullous pemphigoid; biologics; comorbidity; dupilumab; immunosuppressant; real-world study; severity.
Copyright © 2023 Wang, Shan, Li and Zuo.
Conflict of interest statement
Y-GZ served as a speaker for Sanofi, but this was not associated with this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Evaluation of Dupilumab in Patients With Bullous Pemphigoid.JAMA Dermatol. 2023 Sep 1;159(9):953-960. doi: 10.1001/jamadermatol.2023.2428. JAMA Dermatol. 2023. PMID: 37531116 Free PMC article.
-
Long-term efficacy and safety of dupilumab for severe bullous pemphigoid: A prospective cohort study.Int Immunopharmacol. 2023 Dec;125(Pt A):111157. doi: 10.1016/j.intimp.2023.111157. Epub 2023 Nov 3. Int Immunopharmacol. 2023. PMID: 37925949
-
Study Design of a Phase 2/3 Randomized Controlled Trial of Dupilumab in Adults with Bullous Pemphigoid: LIBERTY-BP ADEPT.Adv Ther. 2024 Jul;41(7):2991-3002. doi: 10.1007/s12325-024-02810-3. Epub 2024 Mar 5. Adv Ther. 2024. PMID: 38443648 Free PMC article.
-
Successful treatment of bullous pemphigoid with dupilumab: a case and brief review of the literature.Dermatol Online J. 2021 Apr 15;27(4):13030/qt0dv3f9h6. Dermatol Online J. 2021. PMID: 33999579 Review.
-
Targeting interleukin 4 and interleukin 13: a novel therapeutic approach in bullous pemphigoid.Ann Med. 2023 Dec;55(1):1156-1170. doi: 10.1080/07853890.2023.2188487. Ann Med. 2023. PMID: 36999962 Free PMC article. Review.
Cited by
-
Bullous pemphigoid.Nat Rev Dis Primers. 2025 Feb 20;11(1):12. doi: 10.1038/s41572-025-00595-5. Nat Rev Dis Primers. 2025. PMID: 39979318 Review.
-
Omalizumab and Dupilumab for the Treatment of Bullous Pemphigoid: A Systematic Review.J Clin Med. 2024 Aug 16;13(16):4844. doi: 10.3390/jcm13164844. J Clin Med. 2024. PMID: 39200987 Free PMC article. Review.
-
Recent advances in the genetics and innate immune cells of bullous pemphigoid.Front Immunol. 2025 Jun 18;16:1530407. doi: 10.3389/fimmu.2025.1530407. eCollection 2025. Front Immunol. 2025. PMID: 40607386 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

