A novel 3D spheroid model of rheumatoid arthritis synovial tissue incorporating fibroblasts, endothelial cells, and macrophages
- PMID: 37545512
- PMCID: PMC10402919
- DOI: 10.3389/fimmu.2023.1188835
A novel 3D spheroid model of rheumatoid arthritis synovial tissue incorporating fibroblasts, endothelial cells, and macrophages
Abstract
Objective: Rheumatoid Arthritis (RA) is a progressive and systemic autoimmune disorder associated with chronic and destructive joint inflammation. The hallmarks of joint synovial inflammation are cellular proliferation, extensive neoangiogenesis and infiltration of immune cells, including macrophages. In vitro approaches simulating RA synovial tissue are crucial in preclinical and translational research to evaluate novel diagnostic and/or therapeutic markers. Two-dimensional (2D) settings present very limited in vivo physiological proximity as they cannot recapitulate cell-cell and cell-matrix interactions occurring in the three-dimensional (3D) tissue compartment. Here, we present the engineering of a spheroid-based model of RA synovial tissue which mimics 3D interactions between cells and pro-inflammatory mediators present in the inflamed synovium.
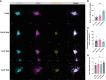
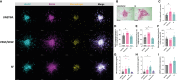
Methods: Spheroids were generated by culturing RA fibroblast-like-synoviocytes (RAFLS), human umbilical vein endothelial cells (ECs) and monocyte-derived macrophages in a collagen-based 3D scaffold. The spheroids were cultured in the presence or absence of vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (bFGF) or RA synovial fluid (SF). Spheroid expansion and cell migration were quantified for all conditions using confocal microscopy and digital image analysis.
Results: A novel approach using machine learning was developed to quantify spheroid outgrowth and used to reexamine the existing spheroid-based model of RA synovial angiogenesis consisting of ECs and RAFLS. A 2-fold increase in the spheroid outgrowth ratio was demonstrated upon VEGF/bFGF stimulation (p<0.05). The addition of macrophages within the spheroid structure (3.75x104 RAFLS, 7.5x104 ECs and 3.0x104 macrophages) resulted in good incorporation of the new cell type. The addition of VEGF/bFGF significantly induced spheroid outgrowth (p<0.05) in the new system. SF stimulation enhanced containment of macrophages within the spheroids.
Conclusion: We present a novel spheroid based model consisting of RAFLS, ECs and macrophages that reflects the RA synovial tissue microenvironment. This model may be used to dissect the role of specific cell types in inflammatory responses in RA, to study specific signaling pathways involved in the disease pathogenesis and examine the effects of novel diagnostic (molecular imaging) and therapeutic compounds, including small molecule inhibitors and biologics.
Keywords: 3D model; endothelial cells; fibroblasts; macrophages; rheumatoid arthritis.
Copyright © 2023 Philippon, van Rooijen, Khodadust, van Hamburg, van der Laken and Tas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Targeting non-canonical nuclear factor-κB signalling attenuates neovascularization in a novel 3D model of rheumatoid arthritis synovial angiogenesis.Rheumatology (Oxford). 2017 Feb;56(2):294-302. doi: 10.1093/rheumatology/kew393. Epub 2016 Nov 17. Rheumatology (Oxford). 2017. PMID: 27864565
-
Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis.Arthritis Res Ther. 2019 Dec 21;21(1):297. doi: 10.1186/s13075-019-2088-3. Arthritis Res Ther. 2019. PMID: 31864394 Free PMC article.
-
STAT3 Mediates the Differential Effects of Oncostatin M and TNFα on RA Synovial Fibroblast and Endothelial Cell Function.Front Immunol. 2019 Aug 28;10:2056. doi: 10.3389/fimmu.2019.02056. eCollection 2019. Front Immunol. 2019. PMID: 31555281 Free PMC article.
-
[Rheumatoid arthritis: new developments in the pathogenesis with special reference to synovial fibroblasts].Z Rheumatol. 2001 Oct;60(5):309-18. doi: 10.1007/s003930170030. Z Rheumatol. 2001. PMID: 11759230 Review. German.
-
Critical Role of Synovial Tissue-Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation.Front Immunol. 2021 Sep 3;12:715894. doi: 10.3389/fimmu.2021.715894. eCollection 2021. Front Immunol. 2021. PMID: 34539648 Free PMC article. Review.
Cited by
-
A Review of Advances in Molecular Imaging of Rheumatoid Arthritis: From In Vitro to Clinic Applications Using Radiolabeled Targeting Vectors with Technetium-99m.Life (Basel). 2024 Jun 12;14(6):751. doi: 10.3390/life14060751. Life (Basel). 2024. PMID: 38929734 Free PMC article. Review.
-
Constructing a 3D co-culture in vitro synovial tissue model for rheumatoid arthritis research.Mater Today Bio. 2025 Jan 24;31:101492. doi: 10.1016/j.mtbio.2025.101492. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39968522 Free PMC article.
-
Emerging Landscape of In Vitro Models for Assessing Rheumatoid Arthritis Management.ACS Pharmacol Transl Sci. 2024 Jul 18;7(8):2280-2305. doi: 10.1021/acsptsci.4c00260. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144547 Free PMC article. Review.
-
Dual-targeted halofuginone hydrobromide nanocomplexes for promotion of macrophage repolarization and apoptosis of rheumatoid arthritis fibroblast-like synoviocytes in adjuvant-induced arthritis in rats.J Pharm Anal. 2024 Nov;14(11):100981. doi: 10.1016/j.jpha.2024.100981. Epub 2024 Apr 22. J Pharm Anal. 2024. PMID: 39703571 Free PMC article.
-
Unveiling the Anti-Angiogenic Potential of Small-Molecule (Kinase) Inhibitors for Application in Rheumatoid Arthritis.Cells. 2025 Jan 11;14(2):102. doi: 10.3390/cells14020102. Cells. 2025. PMID: 39851530 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

