SARS-CoV-2 infection risk is higher in vaccinated patients with inflammatory autoimmune diseases or liver transplantation treated with mycophenolate due to an impaired antiviral immune response: results of the extended follow up of the RIVALSA prospective cohort
- PMID: 37545528
- PMCID: PMC10398576
- DOI: 10.3389/fimmu.2023.1185278
SARS-CoV-2 infection risk is higher in vaccinated patients with inflammatory autoimmune diseases or liver transplantation treated with mycophenolate due to an impaired antiviral immune response: results of the extended follow up of the RIVALSA prospective cohort
Abstract
Background: A relevant proportion of immunocompromised patients did not reach a detectable seroconversion after a full primary vaccination cycle against SARS-CoV-2. The effect of different immunosuppressants and the potential risks for SARS-CoV-2 infection in these subjects is largely unknown.
Methods: Patients from the Rivalsa prospective, observational cohort study with planned anti SARS-CoV-2 third dose mRNA vaccination between October and December 2021 were asked to participate to this follow-up study. Patients were asked about eventual confirmed positivity to SARS-CoV-2 infection within 6 months from the third dose and to undergo a blood draw to evaluate seroconversion status after the additional vaccine shot.
Results: 19 out of 114 patients taking part in the survey developed a confirmed SARS-CoV-2 infection; we identified mycophenolate treatment as an independent predictor of an increased risk of infection even after the third vaccine dose (OR: 5.20, 95% CI: 1.70-20.00, p=0.0053). This result is in agreement with the in vitro evidence that MMF impairs both B and T lymphocytes driven immune responses (reduction both in memory B cells producing anti-spike antibodies and in proliferating CD4+ and CD8+ T cells).
Conclusions: Immunocompromised patients need an additional vaccine administration to reach a detectable seroconversion, thus fostering a more personalized approach to their clinical management. Moreover, patients undergoing mycophenolate treatment show a specific increased infection risk, with respect to other immunosuppressants thus supporting a closer monitoring of their health status.
Keywords: SARS-CoV-2; anti-COVID-19 mRNA vaccination; inflammatory autoimmune diseases; liver transplantation; mycophenolate.
Copyright © 2023 Rizzi, Tonello, Brinno, Zecca, Matino, Cittone, Rizzi, Casciaro, D’Onghia, Colangelo, Minisini, Bellan, Castello, Chiocchetti, Pirisi, Rigamonti, Lilleri, Zavaglio, Bergami, Sola and Sainaghi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
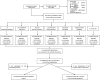

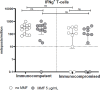

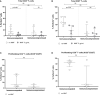
References
-
- Hamm SR, Møller DL, Pérez-Alós K, Hansen CB, Pries-Heje MM, Heftdal LD, et al. Decline in antibody concentration 6 months after two doses of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients and healthy controls. Front Immunol (2022) 13:832501. doi: 10.3389/fimmu.2022.832501 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

