Nickel Complexes of Allyl and Vinyldiphenylphosphine
- PMID: 37545661
- PMCID: PMC10401672
- DOI: 10.1021/acsorginorgau.3c00010
Nickel Complexes of Allyl and Vinyldiphenylphosphine
Abstract
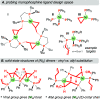
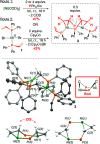
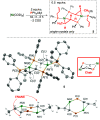
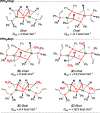
Monodentate phosphine-ligated nickel compounds, e.g., [Ni(PPh3)4] are relevant as active catalysts across a broad range of reactions. This report expands upon the coordination chemistry of this family, offering the reactivity of allyl- and vinyl-substituted diphenylphosphine (PPh2R) with [Ni(COD)2] (COD = 1,5-cyclooctadiene). These reactions provide three-coordinate dinickelacycles that are intermolecularly tethered through adjacent {Ni}-olefin interactions. The ring conformation of such cycles has been studied in the solid-state and using theoretical calculations. Here, a difference in reaction outcome is linked to the presence of an allyl vs vinyl group, where the former is observed to undergo rearrangement, bringing about challenges in clean product isolation.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ananikov V. P. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5 (3), 1964–1971. 10.1021/acscatal.5b00072. - DOI
-
- Greaves M. E.; Johnson Humphrey E. L. B.; Nelson D. J. Reactions of Nickel(0) with Organochlorides, Organobromides, and Organoiodides: Mechanisms and Structure/Reactivity Relationships. Catal. Sci. Technol. 2021, 11 (9), 2980–2996. 10.1039/D1CY00374G. - DOI
-
- Tsou T. T.; Kochi J. K. Mechanism of Oxidative Addition. Reaction of Nickel(0) Complexes with Aromatic Halides. J. Am. Chem. Soc. 1979, 101 (21), 6319–6332. 10.1021/ja00515a028. - DOI
-
- Tolman C. A.; Seidel W. C.; Gosser L. W. Formation of Three-Coordinate Nickel(0) Complexes by Phosphorus Ligand Dissociation from NiL4. J. Am. Chem. Soc. 1974, 96 (1), 53–60. 10.1021/ja00808a009. - DOI
-
- Dalton J.; Foii B. M.; Cassar L.; Ricerche Donegani I. G.; Gerlach D. H.; Kane A. R.; Parshall G. W.; Jesson J. P.; Muetterties E. L.; Hidai M.; Rashiwagi T.; Ireuchi T.; Uchida Y. Oxidative Addition of Aryl Halides to Tris(Triphenylphosphine)Nickel(0). J. Chem. Soc., Dalton Trans. 1975, 7 (23), 2572–2576.
LinkOut - more resources
Full Text Sources
