Comparative phenotype of circulating versus tissue immune cells in human lung and blood compartments during health and disease
- PMID: 37545765
- PMCID: PMC10403752
- DOI: 10.1093/discim/kyad009
Comparative phenotype of circulating versus tissue immune cells in human lung and blood compartments during health and disease
Abstract
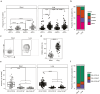
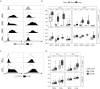
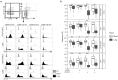
The lung is a dynamic mucosal surface constantly exposed to a variety of immunological challenges including harmless environmental antigens, pollutants, and potentially invasive microorganisms. Dysregulation of the immune system at this crucial site is associated with a range of chronic inflammatory conditions including asthma and Chronic Pulmonary Obstructive Disease (COPD). However, due to its relative inaccessibility, our fundamental understanding of the human lung immune compartment is limited. To address this, we performed flow cytometric immune phenotyping of human lung tissue and matched blood samples that were isolated from 115 donors undergoing lung tissue resection. We provide detailed characterization of the lung mononuclear phagocyte and T cell compartments, demonstrating clear phenotypic differences between lung tissue cells and those in peripheral circulation. Additionally, we show that CD103 expression demarcates pulmonary T cells that have undergone recent TCR and IL-7R signalling. Unexpectedly, we discovered that the immune landscape from asthmatic or COPD donors was broadly comparable to controls. Our data provide a much-needed expansion of our understanding of the pulmonary immune compartment in both health and disease.
Keywords: COPD; T cells; asthma; dendritic cells; mucosal immunology.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
