Proteomic Analysis of Testicular Interstitial Fluid in Men with Azoospermia
- PMID: 37545847
- PMCID: PMC10403685
- DOI: 10.1016/j.euros.2023.06.004
Proteomic Analysis of Testicular Interstitial Fluid in Men with Azoospermia
Abstract
Background: The primary microenvironment of the testis comprises testicular interstitial fluid (TIF) surrounding the seminiferous tubules and testicular interstitial tissue. The pathological alterations of germ and Sertoli cells could affect the TIF composition and might contain putative biomarkers for monitoring active spermatogenesis.
Objective: We identified differentially expressed proteins in the TIF of patients with obstructive (OA) or nonobstructive (NOA) azoospermia to elucidate the underlying etiology of defective spermatogenesis.
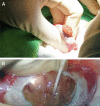
Design setting and participants: We prospectively enrolled nine patients, including three men with OA and six with NOA with (n = 3) and without (n = 3) successful sperm retrieval. Their TIF was collected during the testicular sperm extraction procedure.
Outcome measurements and statistical analysis: TIF was analyzed using liquid chromatography-tandem mass spectrometry to identify differentially expressed proteins specific to OA and NOA with or without successful sperm retrieval. The dysregulated protein was further validated using Western blotting.
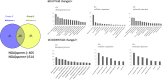
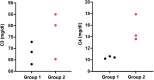
Results and limitations: Among the 555 TIF proteins identified in NOA patients, 14 were downregulated relative to OA patients. These proteins participate in biological processes such as proteolysis, complement activation, and immune responses; complement and coagulation cascade pathways were also enriched. Furthermore, 68 proteins with significantly higher levels were identified in the TIF of NOA patients with successful sperm retrieval than in those with failed sperm retrieval; these are mainly implicated in oxidation-reduction processes. The expression of calreticulin, which can distinguish successful and failed testicular sperm retrieval in the NOA group, was validated by Western blotting.
Conclusions: We provide the first scientific evaluation of TIF protein composition in men with azoospermia. These findings will help identify the physiological and pathological roles of each protein in regulating sperm production. Thus, our study underscores the potential of TIF in sperm retrieval biomarker discovery and would serve as a foundation for further studies to improve treatment strategies against azoospermia.
Patient summary: Using a proteomic approach, we identified and analyzed the total protein content of testicular interstitial fluid in humans with defective spermatogenesis for the first time and discovered altered protein expression patterns in patients with nonobstructive azoospermia (NOA). Proteins related to oxidation-reduction processes were upregulated in NOA patients with successful sperm retrieval compared with those with failed sperm retrieval. This can aid the development of novel diagnostic tools for successful testicular sperm retrieval.
Keywords: Azoospermia; Proteomic; Testicular interstitial fluid.
© 2023 The Author(s).
Figures



Comment in
-
Proteomic-based analysis of testicular interstitial fluid.Nat Rev Urol. 2023 Oct;20(10):578. doi: 10.1038/s41585-023-00822-2. Nat Rev Urol. 2023. PMID: 37684422 No abstract available.
References
-
- Chen Q., Deng T., Han D. Testicular immunoregulation and spermatogenesis. Semin Cell Dev Biol. 2016;59:157–165. - PubMed
-
- Damber J.E., Maddocks S., Widmark A., Bergh A. Testicular blood flow and vasomotion can be maintained by testosterone in Leydig cell-depleted rats. Int J Androl. 1992;15:385–393. - PubMed
-
- Collin O., Bergh A., Damber J.E., Widmark A. Control of testicular vasomotion by testosterone and tubular factors in rats. J Reprod Fertil. 1993;97:115–121. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

