Coordination-directed self-assembly of nano-cages: metal ion-change, ligand-extending, shape-control and transdermal drug delivery
- PMID: 37546215
- PMCID: PMC10401521
- DOI: 10.1039/d3ra04150f
Coordination-directed self-assembly of nano-cages: metal ion-change, ligand-extending, shape-control and transdermal drug delivery
Abstract
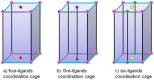
The combination of different pyridyl ligands and metal ions has proven to be a very reliable strategy for controlling the coordination mode of the heterometallic coordination nano-cages. Adjusting the length of the ligands could result in the selective synthesis of several heterometallic coordination nano-cages, either [8Rh + 2M]-4L, [8Rh + 2M]-5L or [8Rh + 4M]-6L cages, derived from the very same precursors (LH3tzdc) through half-sandwich rhodium self-assembly. Moreover, a series of [8Rh + 4M]-6L cages was chosen to exemplify the preparation. The rigidity of various pyridyl donor ligands caused the vertical nano-cage to be energetically preferred and was able to change the self-assembly process through ligand flexibility to selectively give the inclined nano-cage and cross nano-cage.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Heterometallic-Organic Cages with Customized Cavities: Constructed by Bottom-Up Step-Wise Coordination-Driven Self-Assembly.Chemistry. 2024 Nov 12;30(63):e202402499. doi: 10.1002/chem.202402499. Epub 2024 Oct 16. Chemistry. 2024. PMID: 39152769
-
Coordination-Driven Selective Formation of D2 Symmetric Octanuclear Organometallic Cages.Chemistry. 2021 Jul 2;27(37):9524-9528. doi: 10.1002/chem.202101204. Epub 2021 May 17. Chemistry. 2021. PMID: 33882176
-
Single-Step Synthesis of a Heterometallic [Cu2PdL4]2+ Hybrid Metal-Organic Coordination Cage.Angew Chem Int Ed Engl. 2025 Jun 2;64(23):e202506064. doi: 10.1002/anie.202506064. Epub 2025 Apr 17. Angew Chem Int Ed Engl. 2025. PMID: 40167504 Free PMC article.
-
Polyanionic Imido-P(V) Ligands: From Transition Metal Complexes to Coordination Driven Self-Assemblies.Chem Rec. 2022 Mar;22(3):e202100281. doi: 10.1002/tcr.202100281. Epub 2021 Dec 27. Chem Rec. 2022. PMID: 34962082 Review.
-
Coordination-Assembled Molecular Cages with Metal Cluster Nodes.Chem Rec. 2021 Mar;21(3):498-522. doi: 10.1002/tcr.202000130. Epub 2020 Dec 3. Chem Rec. 2021. PMID: 33270374 Review.
Cited by
-
Directed assembly of fullerenols via electrostatic and coordination interactions to fabricate diverse and water-soluble metal cation-fullerene nanocluster complexes.RSC Adv. 2024 Jan 3;14(2):1472-1487. doi: 10.1039/d3ra07725j. eCollection 2024 Jan 2. RSC Adv. 2024. PMID: 38174261 Free PMC article.
References
-
- Beves J. E. Blight B. A. Campbell C. J. Leigh D. A. McBurney R. T. Angew. Chem., Int. Ed. 2011;50:9260–9327. - PubMed
-
- Fujita D. Ueda Y. Sato S. mizuno N. Kumasaka T. Fujita M. Nature. 2016;540:563–566. - PubMed
-
- Sun Q. F. Iwasa J. Ogawa D. Ishido Y. Sato S. Ozeki T. Sei Y. Yamaguchi K. Fujita M. Science. 2010;328:1144–1147. - PubMed
-
- Li M. Li D. O’ Keeffe M. Yaghi O. M. Chem. Rev. 2014;114:1343–1370. - PubMed
LinkOut - more resources
Full Text Sources

