Baveno VII criteria for recompensation predict transplant-free survival in patients with hepatitis B-related decompensated cirrhosis
- PMID: 37546279
- PMCID: PMC10400846
- DOI: 10.1016/j.jhepr.2023.100814
Baveno VII criteria for recompensation predict transplant-free survival in patients with hepatitis B-related decompensated cirrhosis
Abstract
Background & aims: The latest Baveno VII consensus has provided guidance for identifying patients who have truly recompensated from those with hepatic decompensation. This study aimed to evaluate patients' transplant-free survival in three different stages of cirrhosis.
Methods: All patients with chronic HBV infection and liver cirrhosis treated with oral nucleos(t)ide analogues from March 2006 to December 2022 were identified from a territory-wide database in Hong Kong. Patients with follow-up duration of <1 year were excluded. Participants were classified into three mutually exclusive groups: (1) no decompensated events (i.e. compensated group); (2) decompensated events occurred (i.e. decompensated group); or (3) decompensated events occurred followed by recompensation according to Baveno VII criteria (i.e. recompensated group). A time-dependent Cox proportional hazard model was adopted for evaluation. The follow-up period was 5 years.
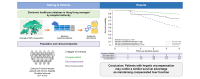
Results: A total of 4,701 patients with cirrhosis and HBV who were treated with entecavir, tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide fumarate (TAF) were identified. During a median follow-up of 5 years (interquartile range 3.7, 5 years), 3,327 (70.8%), 1,347 (29.2%), and 265 (5.6%) patients had compensated, decompensated, and recompensated cirrhosis, respectively, at least once before the end of the study. In the time-dependent multivariable model, the recompensated group had similar transplant-free survival compared with the compensated group (adjusted hazard ratio 1.16; 95% CI 0.72-1.86; p = 0.536). The 5-year transplant-free survival rate was 89.3% for the compensated group, whereas it was 76.0% for the recompensated group, reflecting a minimal difference between the two groups.
Conclusions: The clinical significance of recompensation of cirrhosis in improving patient outcomes for individuals with CHB infection was highlighted in this study. Early identification and treatment with nucleos(t)ide analogues might promote hepatic recompensation and thus reduce mortality in patients with CHB.
Impact and implications: The latest Baveno VII consensus introduces the new concept of hepatic recompensation, which refers to the reversal of the structural and functional changes of cirrhosis after removal, cure, or suppression of the aetiology of cirrhosis. It is essential to investigate the transplant-free survival rates of patients who are able to achieve hepatic recompensation, as this has significant implications for the medical resources required to manage liver failure and transplantation. This study features the clinical significance of hepatic recompensation by comparing patient outcomes of those who achieve it to those who do not. The early identification and use of antiviral treatment with nucleos(t)ide analogues is a pivotal strategy to promote hepatic recompensation, which has the potential to significantly reduce mortality rates in patients with chronic HBV infection and ultimately aid in the elimination of hepatitis.
Keywords: Liver stiffness measurement; Platelet count; Transient elastography; Variceal bleeding.
© 2023 The Author(s).
Conflict of interest statement
GLHW has served as an advisory committee member for Gilead Sciences and Janssen. She has also served as a speaker for Abbvie, Bristol-Myers Squibb, Echosens, Furui, Gilead, and Janssen. VWSW has served as an advisory committee member for 3V-BIO, AbbVie, Allergan, Echosens, Gilead Sciences, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, and Terns; and a speaker for Bristol-Myers Squibb, Echosens, Gilead Sciences, and Merck. He has also received a research grant from Gilead Sciences. HLYC is an advisor for Aligos, Aptorum, Arbutus, Janssen, Gilead, GSK, Roche, Vaccitech, Vir Biotechnology, and Viron Therapeutics; and a speaker for Gilead, Roche, and Viatris. TCFY has served as an advisory committee member and a speaker for Gilead Sciences. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Wong G.L.-H., Chan H.L.-Y., Tse Y.-K., Yip T.C.-F., Lam K.L.-Y., Lui G.C.-Y., et al. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69:793–802. - PubMed
-
- Yip T.C.-F., Wong G.L.-H., Chan H.L.-Y., Tse Y.-K., Lam K.L.-Y., Lui G.C.-Y., et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos (t) ide analogues. J Hepatol. 2019;70:361–370. - PubMed
-
- Peng C.-Y., Chien R.-N., Liaw Y.-F. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57:442–450. - PubMed
-
- Díez C., Berenguer J., Ibañez-Samaniego L., Llop E., Pérez-Latorre L., Catalina M.V., et al. Persistence of clinically significant portal hypertension after eradication of hepatitis C virus in patients with advanced cirrhosis. Clin Infect Dis. 2020;71:2726–2729. - PubMed
LinkOut - more resources
Full Text Sources

