Facile One-Step Pyrolysis of ZnO/Biochar Nanocomposite for Highly Efficient Removal of Methylene Blue Dye from Aqueous Solution
- PMID: 37546599
- PMCID: PMC10398690
- DOI: 10.1021/acsomega.3c01232
Facile One-Step Pyrolysis of ZnO/Biochar Nanocomposite for Highly Efficient Removal of Methylene Blue Dye from Aqueous Solution
Abstract
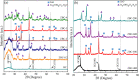
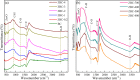
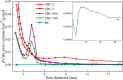
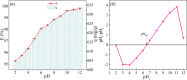
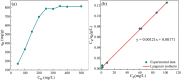
In this work, we developed a facile one-step pyrolysis method for preparing porous ZnO/biochar nanocomposites (ZBCs) with a large surface area to enhance the removal efficiency of dye from aqueous solution. Peanut shells were pyrolyzed under oxygen-limited conditions with a molten salt ZnCl2, which played the roles of the activating agent and precursor for the formation of nanoparticles. The effects of the mass ratio between the molten salt ZnCl2 and peanut shells as well as pyrolysis temperature on the formation of ZBCs were investigated. Characterization results revealed that the as-synthesized ZBCs exhibited a highly porous structure with a specific surface area of 832.12 m2/g, suggesting a good adsorbent for efficient removal of methylene blue (MB). The maximum adsorption capacity of ZBCs on MB was 826.44 mg/g, which surpassed recently reported adsorbents. The formation mechanism of ZnO nanoparticles on the biochar surface was due to ZnCl2 vaporization and reaction with water molecules extracted from the lignocellulosic structures. This study provides a basis for developing a simple and large-scale synthesis method for wastewater with a high adsorption capacity.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Rong Y.; Li S.; Niu J.; Wang Z.; Hao X.; Song C.; Wang T.; Guan G. Carbon-Based Electroactive Ion Exchange Materials: Ultrahigh Removal Efficiency and Ion Selectivity for Rapid Removal of Cs+ Ions. Sep. Purif. Technol. 2021, 274, 11905610.1016/j.seppur.2021.119056. - DOI
-
- Qiu B.; Tao X.; Wang H.; Li W.; Ding X.; Chu H. Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal: A Review. J. Anal. Appl. Pyrolysis 2021, 155, 10508110.1016/j.jaap.2021.105081. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

