This is a preprint.
Reaction hijacking inhibition of Plasmodium falciparum asparagine tRNA synthetase
- PMID: 37546892
- PMCID: PMC10402266
- DOI: 10.21203/rs.3.rs-3198291/v1
Reaction hijacking inhibition of Plasmodium falciparum asparagine tRNA synthetase
Update in
-
Reaction hijacking inhibition of Plasmodium falciparum asparagine tRNA synthetase.Nat Commun. 2024 Jan 31;15(1):937. doi: 10.1038/s41467-024-45224-z. Nat Commun. 2024. PMID: 38297033 Free PMC article.
Abstract
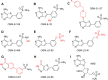
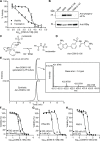
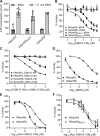
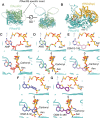
Malaria poses an enormous threat to human health. With ever increasing resistance to currently deployed drugs, breakthrough compounds with novel mechanisms of action are urgently needed. Here, we explore pyrimidine-based sulfonamides as a new low molecular weight inhibitor class with drug-like physical parameters and a synthetically accessible scaffold. We show that the exemplar, OSM-S-106, has potent activity against parasite cultures, low mammalian cell toxicity and low propensity for resistance development. In vitro evolution of resistance using a slow ramp-up approach pointed to the Plasmodium falciparum cytoplasmic asparaginyl tRNA synthetase (PfAsnRS) as the target, consistent with our finding that OSM-S-106 inhibits protein translation and activates the amino acid starvation response. Targeted mass spectrometry confirms that OSM-S-106 is a pro-inhibitor and that inhibition of PfAsnRS occurs via enzyme-mediated production of an Asn-OSM-S-106 adduct. Human AsnRS is much less susceptible to this reaction hijacking mechanism. X-ray crystallographic studies of human AsnRS in complex with inhibitor adducts and docking of pro-inhibitors into a model of Asn-tRNA-bound PfAsnRS provide insights into the structure activity relationship and the selectivity mechanism.
Conflict of interest statement
Competing interests. The authors have no competing interests to declare.
Figures


References
-
- World_Health_Organisation. WHO World Malaria Report 2022. (2022).
-
- Balikagala B, et al. Evidence of artemisinin-resistant malaria in Africa. New England Journal of Medicine 385, 1163–1171 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

