This is a preprint.
TRPV1 controls innate immunity during Citrobacter rodentium enteric infection
- PMID: 37546968
- PMCID: PMC10402119
- DOI: 10.1101/2023.07.26.550772
TRPV1 controls innate immunity during Citrobacter rodentium enteric infection
Update in
-
TRPV1 controls innate immunity during Citrobacter rodentium enteric infection.PLoS Pathog. 2023 Dec 18;19(12):e1011576. doi: 10.1371/journal.ppat.1011576. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38109366 Free PMC article.
Abstract
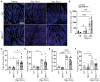
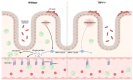
Mucosal immunity is critical to host protection from enteric pathogens and must be carefully controlled to prevent immunopathology. Regulation of immune responses can occur through a diverse range of mechanisms including bi-directional communication with the neurons. Among which include specialized sensory neurons that detect noxious stimuli due to the expression of transient receptor potential vanilloid receptor 1 (TRPV1) ion channel and have a significant role in the coordination of host-protective responses to enteric bacterial pathogens. Here we have used the mouse-adapted attaching and effacing pathogen Citrobacter rodentium to assess the specific role of the TRPV1 channel in coordinating the host response. TRPV1 knockout (TRPV1-/-) mice had a significantly higher C. rodentium burden in the distal colon and fecal pellets compared to wild-type (WT) mice. Increased bacterial burden was correlated with significantly increased colonic crypt hyperplasia and proliferating intestinal epithelial cells in TRPV1-/- mice compared to WT. Despite the increased C. rodentium burden and histopathology, the recruitment of colonic T cells producing IFNγ, IL-17, or IL-22 was similar between TRPV1-/- and WT mice. In evaluating the innate immune response, we identified that colonic neutrophil recruitment in C. rodentium infected TRPV1-/- mice was significantly reduced compared to WT mice; however, this was independent of neutrophil development and maturation within the bone marrow compartment. TRPV1-/- mice were found to have significantly decreased expression of the neutrophil-specific chemokine Cxcl6 and the adhesion molecules Icam1 in the distal colon compared to WT mice. Corroborating these findings, a significant reduction in ICAM-1 and VCAM-1, but not MAdCAM-1 protein on the surface of colonic blood endothelial cells from C. rodentium infected TRPV1-/- mice compared to WT was observed. These findings demonstrate the critical role of TRPV1 in regulating the host protective responses to enteric bacterial pathogens, and mucosal immune responses.
Figures




References
-
- Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125(2):181–95. - PubMed
-
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268(5217):1629–32. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
