Ocular surface disease: a known yet overlooked side effect of topical glaucoma therapy
- PMID: 37547228
- PMCID: PMC10403269
- DOI: 10.3389/ftox.2023.1067942
Ocular surface disease: a known yet overlooked side effect of topical glaucoma therapy
Abstract
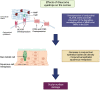
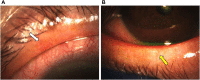
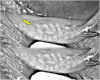
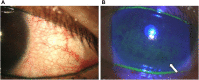
Ocular surface disease (OSD), a disorder affecting the lacrimal and meibomian glands and the corneal and conjunctival epithelium, is a well-known complication of topical glaucoma therapy. OSD can present as a new or pre-existing condition that virtually any anti-glaucoma formulation can exacerbate. As such, both glaucoma and OSD frequently coexist. Typical OSD symptoms include ocular discomfort, redness, burning, and dryness, whereas signs include periorbital and eyelid skin pigmentation, conjunctival scarring, and superficial punctate keratitis. Pressure-lowering eyedrops can cause toxic, allergic, and inflammatory reactions on the ocular surface. The latter can result from either preservatives or direct toxicity from the active molecule. Although usually mild, OSD can cause significant symptoms that lead to poor quality of life, decreased compliance to therapy, glaucoma progression, and worse visual outcomes. Given the chronic nature of glaucoma, lack of curative therapy, and subsequent lifelong treatment, addressing OSD is necessary. This manuscript aims to provide an up-to-date overview of OSD's signs, symptoms, and pathogenic mechanisms from glaucoma therapy toxicity.
Keywords: alpha-adrenergic agonists; beta blockers; carbonic anhydrase inhibitors; dry eye disease; nitric oxide-donating prostaglandin analogs; ocular surface disease; prostaglandin analogs; rho-kinase inhibitors.
Copyright © 2023 Ruiz-Lozano, Azar, Mousa, Quiroga-Garza, Komai, Wheelock-Gutierrez, Cartes and Perez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agnifili L., Mastropasqua R., Fasanella V., Brescia L., Scatena B., Oddone F., et al. (2018). Meibomian gland features and conjunctival goblet cell density in glaucomatous patients controlled with prostaglandin/timolol fixed combinations: A case control, cross-sectional study. J. Glaucoma 27 (4), 364–370. 10.1097/IJG.0000000000000899 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

