Real-life comparison of mortality in patients with SARS-CoV-2 infection at risk for clinical progression treated with molnupiravir or nirmatrelvir plus ritonavir during the Omicron era in Italy: a nationwide, cohort study
- PMID: 37547273
- PMCID: PMC10398591
- DOI: 10.1016/j.lanepe.2023.100684
Real-life comparison of mortality in patients with SARS-CoV-2 infection at risk for clinical progression treated with molnupiravir or nirmatrelvir plus ritonavir during the Omicron era in Italy: a nationwide, cohort study
Abstract
Background: Comparative data on mortality in COVID-19 patients treated with molnupiravir or with nirmatrelvir plus ritonavir are inconclusive. We therefore compared all-cause mortality in community-dwelling COVID-19 patients treated with these drugs during the Omicron era.
Methods: Data collected in the nationwide, population-based, cohort of patients registered in the database of the Italian Medicines Agency (AIFA) were used. To increase completeness of the recorded deaths and date correctness, a cross-check with the National Death Registry provided by the Ministry of the Interior was performed. We included in this study all patients infected by SARS-CoV-2 treated within 5 days after the test date and symptom onset between February 8 and April 30, 2022. All-cause mortalities by day 28 were compared between the two treatment groups after balancing for baseline characteristics using weights obtained from a gradient boosting machine algorithm.
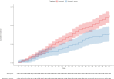
Findings: In the considered timeframe, 17,977 patients treated with molnupiravir and 11,576 patients with nirmatrelvir plus ritonavir were included in the analysis. Most patients (25,617/29,553 = 86.7%) received a full vaccine course including the booster dose. A higher crude incidence rate of all-cause mortality was found among molnupiravir users (51.83 per 100,000 person-days), compared to nirmatrelvir plus ritonavir users (22.29 per 100,000 person-days). However, molnupiravir-treated patients were older than those treated with nirmatrelvir plus ritonavir and differences between the two populations were found as far as types of co-morbidities were concerned. For this reason, we compared the weight-adjusted cumulative incidences using the Aalen estimator and found that the adjusted cumulative incidence rates were 1.23% (95% CI 1.07%-1.38%) for molnupiravir-treated and 0.78% (95% CI 0.58%-0.98%) for nirmatrelvir plus ritonavir-treated patients (adjusted log rank p = 0.0002). Moreover, the weight-adjusted mixed-effect Cox model including Italian regions and NHS centers as random effects and treatment as the only covariate confirmed a significant reduced risk of death in patients treated with nirmatrelvir plus ritonavir. Lastly, a significant reduction in the risk of death associated with nirmatrelvir plus ritonavir was confirmed in patient subgroups, such as in females, fully vaccinated patients, those treated within day 2 since symptom onset and patients without (haemato)-oncological diseases.
Interpretation: Early initiation of nirmatrelvir plus ritonavir was associated for the first time with a significantly reduced risk of all-cause mortality by day 28 compared to molnupiravir, both in the overall population and in patient subgroups, including those fully vaccinated with the booster dose.
Funding: This study did not receive funding.
Keywords: COVID-19; Effectiveness; Molnupiravir; Nirmatrelvir/ritonavir; Real-world; SARS-CoV-2.
© 2023 The Author(s).
Conflict of interest statement
PPO, SC, VS, PR, Giorgio Palù, Carlo Torti, AS, AV, DT, ET, AP, CB, AG, EME, MF, GBB and ML report no competing interests regarding this article. PB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Viiv, Gilead, Jannsen, Merck and Pfizer. CT reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Gilead, Merck, Pfizer, Menarini, GSK, Sanofi, Angelini, thermofischer, Biotest and Diasorin. EN reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Gilead, Eli Lilly, Roche, SOBI. BC reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Angelini, Menarini. Giustino Parruti reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Gilead, Merck, AlphaSigma, Angelini, Pfizer, Lusofarmaco, GSK, Janssen. MB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Angelini, BioMérieux, Cidara, Menarini, MSD, Pfizer and Shionogi. GDP reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from GS, MSD, ViiV, Abbvie, Janssen, GSK, AZ, Pfizer, Roche.
Figures
References
-
- Di Castelnuovo A., Bonaccio M., Costanzo S., et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30:1899–1913. - PMC - PubMed
-
- US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
LinkOut - more resources
Full Text Sources
Miscellaneous