The legacy effect of hyperglycemia and early use of SGLT-2 inhibitors: a cohort study with newly-diagnosed people with type 2 diabetes
- PMID: 37547276
- PMCID: PMC10398589
- DOI: 10.1016/j.lanepe.2023.100666
The legacy effect of hyperglycemia and early use of SGLT-2 inhibitors: a cohort study with newly-diagnosed people with type 2 diabetes
Abstract
Background: A delay in reaching HbA1c targets in patients with newly-diagnosed type 2 diabetes (T2D) is associated with an increased long-term risk of developing cardiovascular diseases (CVD), a phenomenon referred to as legacy effect. Whether an early introduction of glucose-lowering drugs with proven benefit on CVD can attenuate this phenomenon is unknown.
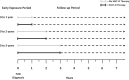
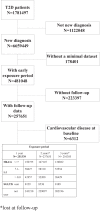
Methods: Using data derived from a large Italian clinical registry, i.e. the AMD Annals, we identified 251,339 subjects with newly-diagnosed T2D and without CVD at baseline. Through Cox regressions adjusted for multiple risk factors, we examined the association between having a mean HbA1c between 7.1 and 8% or >8%, compared with ≤7%, for various periods of early exposure (0-1, 0-2, 0-3 years) and the development of later (mean subsequent follow-up 4.6 ± 2.9 years) CVD, evaluated as a composite of myocardial infarction, stroke, coronary or peripheral revascularization, and coronary or peripheral bypass. We performed this analysis in the overall cohort and then splitting the population in two groups of patients: those that introduced sodium-glucose transport protein 2 inhibitors (SGLT-2i) during the exposure phase and those not treated with these drugs.
Findings: Considering the whole cohort, subjects with both a mean HbA1c between 7.1 and 8% and >8%, compared with patients attaining a mean HbA1c ≤ 7%, showed an increased risk of developing the outcome in all the three early exposure periods assessed, with the highest risk observed in patients with mean HbA1c > 8% in the 3 years exposure period (hazard ratio [HR]1.33; 95% confidence interval [CI] 1.063-1.365). The introduction of SGLT-2i during the exposure periods of 0-1 and 0-2 years eliminated the association between poor glycemic control and the outcome (p for interaction 0.006 and 0.003, respectively, vs. patients with the same degree of glycemic control but not treated with these drugs).
Interpretation: Among patients with newly diagnosed T2D and free of CVD at baseline, a poor glycemic control in the first three years after diagnosis is associated with an increased subsequent risk of CVD. This association is no longer evident when SGLT-2i are introduced in the first two years, suggesting that these drugs attenuate the phenomenon of legacy effect. An early treatment with these drugs might thus promote a long-lasting benefit in patients not attaining proper glycemic control after T2D diagnosis.
Funding: This work was supported, in part, by the Italian Ministry of Health (Ricerca Corrente) to IRCCS MultiMedica.
Keywords: AMD Annals initiative; Cardiovascular diseases; Legacy effect; Metabolic memory; Sodium-glucose cotransporter 2 inhibitors; Type 2 diabetes.
© 2023 The Author(s).
Conflict of interest statement
AC is on the advisory board and does consultancy and lectures for AstraZeneca, BERLIN-CHEMIE, Eli Lilly, Novo Nordisk, Mitsubishi, Roche Diagnostics, and Theras Lifetech. FP is a lecturer for BERLIN-CHEMIE. AN has received honoraria from AstraZeneca, Eli Lilly, Novo Nordisk, and research support from Alfasigma, Novo Nordisk, Sanofi, Shionogi, SOBI. GR is on the advisory board and does consultancy and lectures for Novo Nordisk, Astra Zeneca, Sanofi, Boehringer, Lilly, Mundipharma, and Sanchio. SDC received honoraria for lectures from Eli Lilly, Boehringer, Astra-Zeneca, MundiPharma, MSD, Sanofi, Novo-Nordisk, Daiichi Sankyo, and Bayer. RP received honoraria for lectures from Lilly, Boehringer, AstraZeneca, Novartis, Menarini, MSD, Sanofi, Novo-Nordisk, Vifor, Alfa-Sigma, and Bayer. P.F. reports receiving personal fees from Astra Zeneca, Lilly, Boehringer Ingelheim, Bayer, and Novo Nordisk. The remaining authors declare no conflict of interests.
Figures


References
-
- Rawshani A., Rawshani A., Franzen S., et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. - PubMed
-
- Chalmers J., Cooper M.E. UKPDS and the legacy effect. N Engl J Med. 2008;359(15):1618–1620. - PubMed
-
- Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. - PubMed
LinkOut - more resources
Full Text Sources

