Interaction Between Determinants Governing Urine Volume in Patients With ADPKD on Tolvaptan and its Impact on Quality of Life
- PMID: 37547529
- PMCID: PMC10403673
- DOI: 10.1016/j.ekir.2023.05.011
Interaction Between Determinants Governing Urine Volume in Patients With ADPKD on Tolvaptan and its Impact on Quality of Life
Abstract
Introduction: Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent genetic cause of kidney failure. Tolvaptan, a vasopressin 2 receptor antagonist, is the first drug with proven disease-modifying activity. Long-term treatment adherence is crucial, but a considerable fraction of patients discontinue treatment, because of aquaretic side effects.
Methods: Twenty-four-hour urine was collected in 75 patients with ADPKD during up-titration of tolvaptan and, in combination with clinical characteristics, examined to identify factors influencing urine volume. Patient-reported outcomes were analyzed using the Short Form-12 (SF-12) and patient-reported outcomes questionnaires reporting micturition frequency and burden of urine volume.
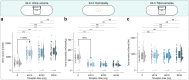
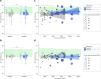
Results: Initiation of therapy led to a large increase in urine volume followed by only minor further increase during up-dosing. Younger patients and patients with better kidney function experienced a larger relative rise. Twenty-four-hour urine osmolality dropped by about 50% after therapy initiation independently of dose, with a considerable proportion of patients achieving adequate suppression. Sodium and potassium intake turned out to be the only significant modifiable factors for urine volume after multivariate linear regression models, whereas age and weight could be identified as non-modifiable factors. No change in quality of life (QoL) was detected in relation to treatment or urine volume using SF-12 questionnaires, a finding that was further supported by the results of the patient-reported outcomes assessment.
Conclusion: This study provides an in-detail analysis of factors associated with the degree of polyuria on tolvaptan and puts them into the context of QoL. These findings will contribute to optimized patient counseling regarding this treatment option in ADPKD.
Keywords: ADPKD; dose; osmolality; quality of life; tolvaptan; urine volume.
© 2023 Published by Elsevier Inc. on behalf of the International Society of Nephrology.
Figures



References
-
- Spithoven E.M., Kramer A., Meijer E., et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival--an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(suppl 4):iv15–iv25. doi: 10.1093/ndt/gfu017. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

