Phenylacetylglutamine and trimethylamine N-oxide: Two uremic players, different actions
- PMID: 37548021
- PMCID: PMC10909455
- DOI: 10.1111/eci.14074
Phenylacetylglutamine and trimethylamine N-oxide: Two uremic players, different actions
Abstract
Background: Chronic kidney disease (CKD) patients exhibit a heightened cardiovascular (CV) risk which may be partially explained by increased medial vascular calcification. Although gut-derived uremic toxin trimethylamine N-oxide (TMAO) is associated with calcium-phosphate deposition, studies investigating phenylacetylglutamine's (PAG) pro-calcifying potential are missing.
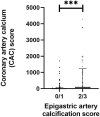
Methods: The effect of TMAO and PAG in vascular calcification was investigated using 120 kidney failure patients undergoing living-donor kidney transplantation (LD-KTx), in an observational, cross-sectional manner. Uremic toxin concentrations were related to coronary artery calcification (CAC) score, epigastric artery calcification score, and markers of established non-traditional risk factors that constitute to the 'perfect storm' that drives early vascular aging in this patient population. Vascular smooth muscle cells were incubated with TMAO or PAG to determine their calcifying effects in vitro and analyse associated pathways by which these toxins may promote vascular calcification.
Results: TMAO, but not PAG, was independently associated with CAC score after adjustment for CKD-related risk factors in kidney failure patients. Neither toxin was associated with epigastric artery calcification score; however, PAG was independently, positively associated with 8-hydroxydeoxyguanosine. Similarly, TMAO, but not PAG, promoted calcium-phosphate deposition in vitro, while both uremic solutes induced oxidative stress.
Conclusions: In conclusion, our translational data confirm TMAO's pro-calcifying effects, but both toxins induced free radical production detrimental to vascular maintenance. Our findings suggest these gut-derived uremic toxins have different actions on the vessel wall and therapeutically targeting TMAO may help reduce CV-related mortality in CKD.
Keywords: TMAO; kidney failure; oxidative stress; phenylacetylglutamine; vascular calcification.
© 2023 The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
Conflict of interest statement
P.S. reports serving in advisory or leadership roles for Astellas, AstraZeneca, Baxter, Fresenius Medical Care (FMC), GlaxoSmithKline, Reata and Vifor; receiving honoraria from Astellas, AstraZeneca, Baxter, FMC, Novo Nordisk, Boerhinger Ingelheim and Pfizer/Bristol Square Myers (BSM); receiving research funding from AstraZeneca and Bayer; serving on advisory boards for AstraZeneca, Baxter, FMC, GlaxoSmithKline, Reata and Vifor. All other authors of this manuscript have nothing to declare.
Figures





References
-
- Kooman JP, Kotanko P, Schols AMWJ, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10:732‐742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

