Experimental NOE, Chemical Shift, and Proline Isomerization Data Provide Detailed Insights into Amelotin Oligomerization
- PMID: 37548612
- PMCID: PMC10436275
- DOI: 10.1021/jacs.3c05710
Experimental NOE, Chemical Shift, and Proline Isomerization Data Provide Detailed Insights into Amelotin Oligomerization
Abstract
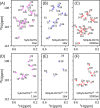
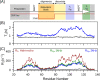
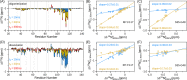
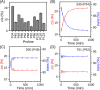
Amelotin is an intrinsically disordered protein (IDP) rich in Pro residues and is involved in hydroxyapatite mineralization. It rapidly oligomerizes under physiological conditions of pH and pressure but reverts to its monomeric IDP state at elevated pressure. We identified a 105-residue segment of the protein that becomes ordered upon oligomerization, and we used pressure-jump NMR spectroscopy to measure long-range NOE contacts that exist exclusively in the oligomeric NMR-invisible state. The kinetics of oligomerization and dissociation were probed at the residue-specific level, revealing that the oligomerization process is initiated in the C-terminal half of the segment. Using pressure-jump NMR, the degree of order in the oligomer at the sites of Pro residues was probed by monitoring changes in cis/trans equilibria relative to the IDP state after long-term equilibration under oligomerizing conditions. Whereas most Pro residues revert to trans in the oligomeric state, Pro-49 favors a cis configuration and three Pro residues retain an unchanged cis fraction, pointing to their local lack of order in the oligomeric state. NOE contacts and secondary 13C chemical shifts in the oligomeric state indicate the presence of an 11-residue α-helix, preceded by a small intramolecular antiparallel β-sheet, with slower formation of long-range intermolecular interactions to N-terminal residues. Although none of the models generated by AlphaFold2 for the amelotin monomer was consistent with experimental data, subunits of a hexamer generated by AlphaFold-Multimer satisfied intramolecular NOE and chemical shift data and may provide a starting point for developing atomic models for the oligomeric state.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Foguel D.; Suarez M. C.; Ferrao-Gonzales A. D.; Porto T. C. R.; Palmieri L.; Einsiedler C. M.; Andrade L. R.; Lashuel H. A.; Lansbury P. T.; Kelly J. W.; Silva J. L. Dissociation of amyloid fibrils of alpha-synuclein and transthyretin by pressure reveals their reversible nature and the formation of water-excluded cavities. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (17), 9831–9836. 10.1073/pnas.1734009100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

