Immunoglobulin A Antibodies Against Myelin Oligodendrocyte Glycoprotein in a Subgroup of Patients With Central Nervous System Demyelination
- PMID: 37548987
- PMCID: PMC10407763
- DOI: 10.1001/jamaneurol.2023.2523
Immunoglobulin A Antibodies Against Myelin Oligodendrocyte Glycoprotein in a Subgroup of Patients With Central Nervous System Demyelination
Erratum in
-
Error in Figure 2B.JAMA Neurol. 2024 Jan 1;81(1):88. doi: 10.1001/jamaneurol.2023.4587. JAMA Neurol. 2024. PMID: 37955904 Free PMC article. No abstract available.
Abstract
Importance: Differential diagnosis of patients with seronegative demyelinating central nervous system (CNS) disease is challenging. In this regard, evidence suggests that immunoglobulin (Ig) A plays a role in the pathogenesis of different autoimmune diseases. Yet little is known about the presence and clinical relevance of IgA antibodies against myelin oligodendrocyte glycoprotein (MOG) in CNS demyelination.
Objective: To investigate the frequency of MOG-IgA and associated clinical features in patients with demyelinating CNS disease and healthy controls.
Design, setting, and participants: This longitudinal study comprised 1 discovery and 1 confirmation cohort derived from 5 centers. Participants included patients with suspected or confirmed demyelinating diseases and healthy controls. MOG-IgA, MOG-IgG, and MOG-IgM were measured in serum samples and cerebrospinal fluid (CSF) of patients, who were assessed from September 2012 to April 2022.
Main outcomes and measures: Frequency and clinical features of patients who were seropositive for MOG-IgA and double-seronegative for aquaporin 4 (AQP4) IgG and MOG-IgG.
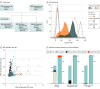
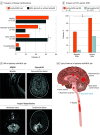
Results: After the exclusion of 5 participants with coexisting AQP4-IgG and MOG-IgA, MOG-IgG, and/or MOG-IgM, 1339 patients and 110 healthy controls were included; the median follow-up time was 39 months (range, 0-227 months). Of included patients with isolated MOG-IgA, 11 of 18 were female (61%), and the median age was 31.5 years (range, 3-76 years). Among patients double-seronegative for AQP4-IgG and MOG-IgG (1126/1339; 84%), isolated MOG-IgA was identified in 3 of 50 patients (6%) with neuromyelitis optica spectrum disorder, 5 of 228 patients (2%) with other CNS demyelinating diseases, and 10 of 848 patients (1%) with multiple sclerosis but in none of the healthy controls (0/110). The most common disease manifestation in patients seropositive for isolated MOG-IgA was myelitis (11/17 [65%]), followed by more frequent brainstem syndrome (7/16 [44%] vs 14/75 [19%], respectively; P = .048), and infrequent manifestation of optic neuritis (4/15 [27%] vs 46/73 [63%], respectively; P = .02) vs patients with MOG-IgG. Among patients fulfilling 2017 McDonald criteria for multiple sclerosis, MOG-IgA was associated with less frequent CSF-specific oligoclonal bands (4/9 [44%] vs 325/351 [93%], respectively; P < .001) vs patients with multiple sclerosis who were MOG-IgG/IgA seronegative. Further, most patients with isolated MOG-IgA presented clinical attacks after recent infection or vaccination (7/11 [64%]).
Conclusion and relevance: In this study, MOG-specific IgA was identified in a subgroup of patients who were double-seronegative for AQP4-/MOG-IgG, suggesting that MOG-IgA may be a novel diagnostic biomarker for patients with CNS demyelination.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

