Vertical transmission of African-lineage Zika virus through the fetal membranes in a rhesus macaque (Macaca mulatta) model
- PMID: 37549143
- PMCID: PMC10434957
- DOI: 10.1371/journal.ppat.1011274
Vertical transmission of African-lineage Zika virus through the fetal membranes in a rhesus macaque (Macaca mulatta) model
Abstract
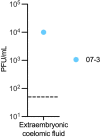
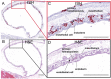
Zika virus (ZIKV) can be transmitted vertically from mother to fetus during pregnancy, resulting in a range of outcomes including severe birth defects and fetal/infant death. Potential pathways of vertical transmission in utero have been proposed but remain undefined. Identifying the timing and routes of vertical transmission of ZIKV may help us identify when interventions would be most effective. Furthermore, understanding what barriers ZIKV overcomes to effect vertical transmission may help improve models for evaluating infection by other pathogens during pregnancy. To determine the pathways of vertical transmission, we inoculated 12 pregnant rhesus macaques with an African-lineage ZIKV at gestational day 30 (term is 165 days). Eight pregnancies were surgically terminated at either seven or 14 days post-maternal infection. Maternal-fetal interface and fetal tissues and fluids were collected and evaluated for ZIKV using RT-qPCR, in situ hybridization, immunohistochemistry, and plaque assays. Four additional pregnant macaques were inoculated and terminally perfused with 4% paraformaldehyde at three, six, nine, or ten days post-maternal inoculation. For these four cases, the entire fixed pregnant uterus was evaluated with in situ hybridization for ZIKV RNA. We determined that ZIKV can reach the MFI by six days after infection and infect the fetus by ten days. Infection of the chorionic membrane and the extraembryonic coelomic fluid preceded infection of the fetus and the mesenchymal tissue of the placental villi. We did not find evidence to support a transplacental route of ZIKV vertical transmission via infection of syncytiotrophoblasts or villous cytotrophoblasts. The pattern of infection observed in the maternal-fetal interface provides evidence of paraplacental vertical ZIKV transmission through the chorionic membrane, the outer layer of the fetal membranes.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures












Comment in
-
Decidual leukocytes respond to African lineage Zika virus infection with mild anti-inflammatory changes during acute infection in rhesus macaques.Front Immunol. 2024 Mar 7;15:1363169. doi: 10.3389/fimmu.2024.1363169. eCollection 2024. Front Immunol. 2024. PMID: 38515747 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

