Cross-frequency coupling between slow harmonics via the real brainstem oscillators: An in vivo animal study
- PMID: 37549170
- PMCID: PMC10406189
- DOI: 10.1371/journal.pone.0289657
Cross-frequency coupling between slow harmonics via the real brainstem oscillators: An in vivo animal study
Abstract
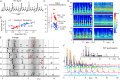
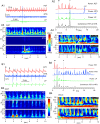
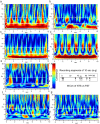
Brain waves of discrete rhythms (gamma to delta frequency ranges) are ubiquitously recorded and interpreted with respect to probable corresponding specific functions. The most challenging idea of interpreting varied frequencies of brain waves has been postulated as a communication mechanism in which different neuronal assemblies use specific ranges of frequencies cooperatively. One promising candidate is cross-frequency coupling (CFC), in which some neuronal assemblies efficiently utilize the fastest gamma range brain waves as an information carrier (phase-amplitude CFC); however, phase-phase CFC via the slowest delta and theta waves has rarely been described to date. Moreover, CFC has rarely been reported in the animal brainstem including humans, which most likely utilizes the slowest waves (delta and theta ranges). Harmonic waves are characterized by the presence of a fundamental frequency with several overtones, multiples of the fundamental frequency. Rat brainstem waves seemed to consist of slow harmonics with different frequencies that could cooperatively produce a phase-phase CFC. Harmonic rhythms of different frequency ranges can cross-couple with each other to sustain robust and resilient consonance via real oscillators, notwithstanding any perturbations.
Copyright: © 2023 Yoshinori Kawai. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Cerebral Spreading Depression Transient Disruption of Cross-Frequency Coupling in the Rat Brain: Preliminary Observations.Adv Exp Med Biol. 2021;1269:209-216. doi: 10.1007/978-3-030-48238-1_33. Adv Exp Med Biol. 2021. PMID: 33966219
-
Reduction in LFP cross-frequency coupling between theta and gamma rhythms associated with impaired STP and LTP in a rat model of brain ischemia.Front Comput Neurosci. 2013 Apr 5;7:27. doi: 10.3389/fncom.2013.00027. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23576981 Free PMC article.
-
Complex interplay between spectral harmonicity and different types of cross-frequency couplings in nonlinear oscillators and biologically plausible neural network models.Phys Rev E. 2020 Dec;102(6-1):062401. doi: 10.1103/PhysRevE.102.062401. Phys Rev E. 2020. PMID: 33466042
-
Working memory and neural oscillations: α-γ versus θ-γ codes for distinct WM information?Trends Cogn Sci. 2014 Jan;18(1):16-25. doi: 10.1016/j.tics.2013.10.010. Epub 2013 Nov 19. Trends Cogn Sci. 2014. PMID: 24268290 Review.
-
Phase correlation among rhythms present at different frequencies: spectral methods, application to microelectrode recordings from visual cortex and functional implications.Int J Psychophysiol. 1997 Jun;26(1-3):171-89. doi: 10.1016/s0167-8760(97)00763-0. Int J Psychophysiol. 1997. PMID: 9203002 Review.
Cited by
-
The brain-heart axis: integrative cooperation of neural, mechanical and biochemical pathways.Nat Rev Cardiol. 2025 Aug;22(8):537-550. doi: 10.1038/s41569-025-01140-3. Epub 2025 Mar 3. Nat Rev Cardiol. 2025. PMID: 40033035 Review.
References
-
- Buzsaki G. Rhythms of the Brain. New York: Oxford University Press, Inc.; 2006.
MeSH terms
LinkOut - more resources
Full Text Sources

