Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway
- PMID: 37549281
- PMCID: PMC10438385
- DOI: 10.1073/pnas.2303809120
Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway
Abstract
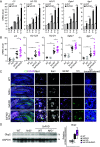
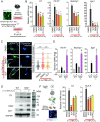
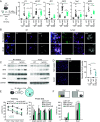
Neuroinflammation is a common feature of neurodegenerative disorders such as Alzheimer's disease (AD). Neuroinflammation is induced by dysregulated glial activation, and astrocytes, the most abundant glial cells, become reactive upon neuroinflammatory cytokines released from microglia and actively contribute to neuronal loss. Therefore, blocking reactive astrocyte functions is a viable strategy to manage neurodegenerative disorders. However, factors or therapeutics directly regulating astrocyte subtypes remain unexplored. Here, we identified transcription factor NF-E2-related factor 2 (Nrf2) as a therapeutic target in neurotoxic reactive astrocytes upon neuroinflammation. We found that the absence of Nrf2 promoted the activation of reactive astrocytes in the brain tissue samples obtained from AD model 5xFAD mice, whereas enhanced Nrf2 expression blocked the induction of reactive astrocyte gene expression by counteracting NF-κB subunit p65 recruitment. Neuroinflammatory astrocytes robustly up-regulated genes associated with type I interferon and the antigen-presenting pathway, which were suppressed by Nrf2 pathway activation. Moreover, impaired cognitive behaviors observed in AD mice were rescued upon ALGERNON2 treatment, which potentiated the Nrf2 pathway and reduced the induction of neurotoxic reactive astrocytes. Thus, we highlight the potential of astrocyte-targeting therapy by promoting the Nrf2 pathway signaling for neuroinflammation-triggered neurodegeneration.
Keywords: Nrf2; neuroinflammation; neurotoxic reactive astrocytes.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

