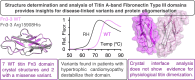
Structure determination and analysis of titin A-band fibronectin type III domains provides insights for disease-linked variants and protein oligomerisation
- PMID: 37549721
- PMCID: PMC10862085
- DOI: 10.1016/j.jsb.2023.108009
Structure determination and analysis of titin A-band fibronectin type III domains provides insights for disease-linked variants and protein oligomerisation
Abstract
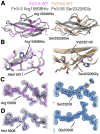
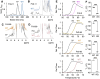
Titin is the largest protein found in nature and spans half a sarcomere in vertebrate striated muscle. The protein has multiple functions, including in the organisation of the thick filament and acting as a molecular spring during the muscle contraction cycle. Missense variants in titin have been linked to both cardiac and skeletal myopathies. Titin is primarily composed of tandem repeats of immunoglobulin and fibronectin type III (Fn3) domains in a variety of repeat patterns; however, the vast majority of these domains have not had their high-resolution structure determined experimentally. Here, we present the crystal structures of seven wild type titin Fn3 domains and two harbouring rare missense variants reported in hypertrophic cardiomyopathy (HCM) patients. All domains present the typical Fn3 fold, with the domains harbouring variants reported in HCM patients retaining the wild-type conformation. The effect on domain folding and stability were assessed for five rare missense variants found in HCM patients: four caused thermal destabilization of between 7 and 13 °C and one prevented the folding of its domain. The structures also allowed us to locate the positions of residues whose mutations have been linked to congenital myopathies and rationalise how they convey their deleterious effects. We find no evidence of physiological homodimer formation, excluding one hypothesised mechanism as to how titin variants could exert pathological effects.
Keywords: Fibronectin type III; Muscle; Myopathy; Titin; X-ray crystallography.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Amodeo P., Fraternali F., Lesk A.M., Pastore A. Modularity and homology: modelling of the titin type I modules and their interfaces. J. Mol. Biol. 2001;311:283–296. - PubMed
-
- Arimura T., Bos J.M., Sato A., Kubo T., Okamoto H., Nishi H., Harada H., Koga Y., Moulik M., Doi Y.L., Towbin J.A., Ackerman M.J., Kimura A. Cardiac Ankyrin Repeat Protein Gene (ANKRD1) Mutations in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2009;54:334–342. - PubMed
-
- Bennett P.M., Gautel M. Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament. J. Mol. Biol. 1996;259:896–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

