Characterization of a novel inhibitor for the New Delhi metallo-β-lactamase-4: Implications for drug design and combating bacterial drug resistance
- PMID: 37549809
- PMCID: PMC10514461
- DOI: 10.1016/j.jbc.2023.105135
Characterization of a novel inhibitor for the New Delhi metallo-β-lactamase-4: Implications for drug design and combating bacterial drug resistance
Abstract
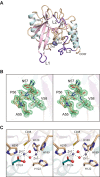
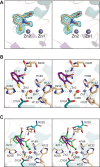
The bacterial metallo-β-lactamases (MBLs) catalyze the inactivation of β-lactam antibiotics. Identifying novel pharmacophores remains crucial for the clinical development of additional MBL inhibitors. Previously, 1-hydroxypyridine-2(1H)-thione-6-carboxylic acid, hereafter referred to as 1,2-HPT-6-COOH, was reported as a low cytotoxic nanomolar β-lactamase inhibitor of Verona-integron-encoded metallo-β-lactamase 2, capable of rescuing β-lactam antibiotic activity. In this study, we explore its exact mechanism of inhibition and the extent of its activity through structural characterization of its binding to New Delhi metallo-β-lactamase 4 (NDM-4) and its inhibitory activity against both NDM-1 and NDM-4. Of all the structure-validated MBL inhibitors available, 1,2-HPT-6-COOH is the first discovered compound capable of forming an octahedral coordination sphere with Zn2 of the binuclear metal center. This unexpected mechanism of action provides important insight for the further optimization of 1,2-HPT-6-COOH and the identification of additional pharmacophores for MBL inhibition.
Keywords: antibiotic resistance; antibiotics; computational biology; crystallography; enzyme kinetics; enzyme structure.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. Influenzae. Br. J. Exp. Pathol. 1929;10:226–236.
-
- Abraham E.P., Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146:837. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

