Auricularia auricula polysaccharides attenuate obesity in mice through gut commensal Papillibacter cinnamivorans
- PMID: 37549868
- PMCID: PMC10555930
- DOI: 10.1016/j.jare.2023.08.003
Auricularia auricula polysaccharides attenuate obesity in mice through gut commensal Papillibacter cinnamivorans
Abstract
Introduction: Auricularia auricula is a well-known traditional edible and medical fungus with high nutritional and pharmacological values, as well as metabolic and immunoregulatory properties. Nondigestible fermentable polysaccharides are identified as primary bioactive constituents of Auricularia auricula extracts. However, the exact mechanisms underlying the effects of Auricularia auricula polysaccharides (AAP) on obesity and related metabolic endpoints, including the role of the gut microbiota, remain insufficiently understood.
Methods: The effects of AAP on obesity were assessed within high-fat diet (HFD)-based mice through obesity trait analysis and metabolomic profiling. To determine the mechanistic role of the gut microbiota in observed anti-obesogenic effects AAP, faecal microbiota transplantation (FMT) and pseudo-germ-free mice model treated with antibiotics were also applied, together with 16S rRNA genomic-derived taxonomic profiling.
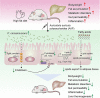
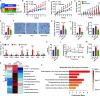
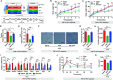
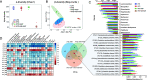
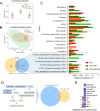
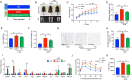
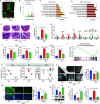
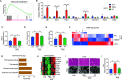
Results: High-fat diet (HFD) murine exposure to AAP thwarted weight gains, reduced fat depositing and enhanced glucose tolerance, together with upregulating thermogenesis proteomic biomarkers within adipose tissue. Serum metabolome indicated these effects were associated with changes in fatty acid metabolism. Intestine-dwelling microbial population assessments discovered that AAP selectively enhanced Papillibacter cinnamivorans, a commensal bacterium with reduced presence in HFD mice. Notably, HFD mice treated with oral formulations of P. cinnamivorans attenuated obesity, which was linked to decreased intestinal lipid transportation and hepatic thermogenesis. Mechanistically, it was demonstrated that P. cinnamivorans regulated intestinal lipids metabolism and liver thermogenesis by reducing the proinflammatory response and gut permeability in a JAK-STAT signaling-related manner.
Conclusion: Datasets from the present study show that AAP thwarted dietary-driven obesity and metabolism-based disorders by regulating intestinal lipid transportation, a mechanism that is dependent on the gut commensal P. cinnamivorans. These results indicated AAP and P. cinnamivorans as newly identified pre- and probiotics that could serve as novel therapeutics against obesity.
Keywords: Auricularia auricula; Gut microbiota; Obesity; Papillibacter cinnamivorans; Polysaccharides.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Effects of Auricularia auricula Polysaccharides on Gut Microbiota Composition in Type 2 Diabetic Mice.Molecules. 2022 Sep 16;27(18):6061. doi: 10.3390/molecules27186061. Molecules. 2022. PMID: 36144789 Free PMC article.
-
Auricularia auricula-judae (Bull.) polysaccharides improve type 2 diabetes in HFD/STZ-induced mice by regulating the AKT/AMPK signaling pathways and the gut microbiota.J Food Sci. 2021 Dec;86(12):5479-5494. doi: 10.1111/1750-3841.15963. Epub 2021 Nov 17. J Food Sci. 2021. PMID: 34787328
-
Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis.Gut. 2019 Feb;68(2):248-262. doi: 10.1136/gutjnl-2017-315458. Epub 2018 Jul 14. Gut. 2019. PMID: 30007918
-
Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities.Carbohydr Polym. 2020 Nov 1;247:116750. doi: 10.1016/j.carbpol.2020.116750. Epub 2020 Jul 13. Carbohydr Polym. 2020. PMID: 32829866 Review.
-
The Current State and Future Prospects of Auricularia auricula's Polysaccharide Processing Technology Portfolio.Molecules. 2023 Jan 6;28(2):582. doi: 10.3390/molecules28020582. Molecules. 2023. PMID: 36677640 Free PMC article. Review.
Cited by
-
Interactions between Gut Microbiota and Natural Bioactive Polysaccharides in Metabolic Diseases: Review.Nutrients. 2024 Aug 24;16(17):2838. doi: 10.3390/nu16172838. Nutrients. 2024. PMID: 39275156 Free PMC article. Review.
-
High-dose thiamine supplementation ameliorates obesity induced by a high-fat and high-fructose diet in mice by reshaping gut microbiota.Front Nutr. 2025 Feb 7;12:1532581. doi: 10.3389/fnut.2025.1532581. eCollection 2025. Front Nutr. 2025. PMID: 39990607 Free PMC article.
-
Assessment of the Safety and Probiotic Properties of Enterococcus faecium B13 Isolated from Fermented Chili.Microorganisms. 2024 May 15;12(5):994. doi: 10.3390/microorganisms12050994. Microorganisms. 2024. PMID: 38792822 Free PMC article.
-
Prickly ash seeds can promote healthy production of sheep by regulating the rumen microbial community.Front Microbiol. 2024 May 20;15:1364517. doi: 10.3389/fmicb.2024.1364517. eCollection 2024. Front Microbiol. 2024. PMID: 38832114 Free PMC article.
-
The microbiome's influence on obesity: mechanisms and therapeutic potential.Sci China Life Sci. 2025 Mar;68(3):657-672. doi: 10.1007/s11427-024-2759-3. Epub 2024 Nov 28. Sci China Life Sci. 2025. PMID: 39617855 Review.
References
-
- Sommer F., Backhed F. The gut microbiota - masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–238. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

