Utility of Protein Microarrays for Detection of Classified and Novel Antibodies in Autoimmune Neurologic Disease
- PMID: 37550073
- PMCID: PMC10406426
- DOI: 10.1212/NXI.0000000000200145
Utility of Protein Microarrays for Detection of Classified and Novel Antibodies in Autoimmune Neurologic Disease
Abstract
Background and objectives: Neural antibodies are detected by tissue-based indirect immunofluorescence assay (IFA) in Mayo Clinic's Neuroimmunology Laboratory practice, but the process of characterizing and validating novel antibodies is lengthy. We report our assessment of human protein arrays.
Methods: Assessment of arrays (81% human proteome coverage) was undertaken using diverse known positive samples (17 serum and 14 CSF). Samples from patients with novel neural antibodies were reflexed from IFA to arrays. Confirmatory assays were cell-based (CBA) or line blot. Epitope mapping was undertaken using phage display immunoprecipitation sequencing (PhiPSeq).
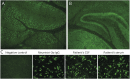
Results: Control positive samples known to be reactive with linear epitopes of intracellular antigens (e.g., ANNA-1 [anti-Hu]) were readily identified by arrays in 20 of 21 samples. By contrast, 10 positive controls known to be enriched with antibodies against cell surface protein conformational epitopes (e.g., GluN1 subunit of NMDA-R) were indistinguishable from background signal. Three antibodies, previously characterized by other investigators (but unclassified in our laboratory), were unmasked in 4 patients using arrays (July-December 2022): Neurexin-3α, 1 patient; regulator of gene protein signaling (RGS)8, 1 patient; and seizure-related homolog like 2 (SEZ6L2), 2 patients. All were accompanied by previously reported phenotypes (encephalitis, 1; cerebellar ataxia, 3). Patient 1 had subacute onset of seizures and encephalopathy. Neurexin-3α ranked high in CSF (second ranked neural protein) but low in serum (660th overall). Neurexin-3α CBA was positive in both samples. Patient 2 presented with rapidly progressive cerebellar ataxia. RGS8 ranked the highest neural protein in available CSF sample by array (third overall). RGS8-specific line blot was positive. Patients 3 and 4 had rapidly progressive cerebellar ataxia. SEZ6L2 was the highest ranked neural antigen by arrays in all samples (CSF, 1, serum, 2; Patient 3, ranked 9th overall in CSF, 11th in serum; Patient 4, 6th overall in serum]). By PhIPSeq, diverse neurexin-3α epitopes (including cell surface) were detected in CSF from patient 1, but no SEZ6L2 peptides were detected for serum or CSF samples from Patient 3.
Discussion: Individualized autoimmune neurologic diagnoses may be accelerated using protein arrays. They are optimal for detection of intracellular antigen-reactive antibodies, though certain cell surface-directed antibodies (neurexin-3α and SEZ6L2) may also be detected.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
A. McKeon reports research funding from NIH (NIH: RO1NS126227, U01NS120901), patents issued for GFAP and MAP1B-IgGs and patents pending for PDE10A, Septins-5 and -7, and KLCHL11-IgGs, and has consulted for Janssen and Roche pharmaceuticals, without personal compensation. C. Lesnick reports no disclosures relevant to the manuscript. N. Vorasoot reports no disclosures relevant to the manuscript. M.W. Buckley reports no disclosures relevant to the manuscript. S. Dasari reports no disclosures relevant to the manuscript. E.P. Flanagan has funding from NIH (R01NS113828), has served on advisory boards for Alexion, Genentech, Horizon Therapeutics and UCB, has received honoraria from Pharmacy Times and UpToDate, and has a patent pending for DACH1-IgG as a biomarker of paraneoplastic autoimmunity; M. Gilligan reports no disclosures relevant to the manuscript. R. Lafrance Corey reports no disclosures relevant to the manuscript. R. Miske is employed by Euroimmun. S.J. Pittock is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker, has patents pending for KLHL11-IgG and Septin-5-IgG, and issued for MAP1B-IgG as markers of neurologic autoimmunity and paraneoplastic disorders, has consulted for Alexion and Medimmune; and has received research support from Genentech, Grifols, Medimmune, and Alexion; M Scharf is employed by Euroimmun. B. Yang reports no disclosures relevant to this manuscript. A. Zekeridou has patent applications pending on PDE10A-IgG and DACH1-IgG as biomarkers of paraneoplastic neurologic autoimmunity and has received research funding from Genentech; D. Dubey has research support from Department of Defence (CA210208), Centers of Multiple Sclerosis and Autoimmune Neurology, and Clinical and Translational Science, Mayo Clinic, and Grifols pharmaceuticals, has consulted for UCB, Immunovant, Argenx and Astellas pharmaceuticals (compensation for consulting activities paid directly to Mayo Clinic), and has patents pending for KLHL11-IgG, LUZP4-IgG, and cavin-4-IgG as markers of neurologic autoimmunity; J Mills reports no disclosures relevant to the manuscript. Go to
Figures


References
-
- Altermatt HJ, Rodriguez M, Scheithauer BW, Lennon VA. Paraneoplastic anti-Purkinje and type I anti-neuronal nuclear autoantibodies bind selectively to central, peripheral, and autonomic nervous system cells. Lab Invest. 1991;65(4):412-420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
